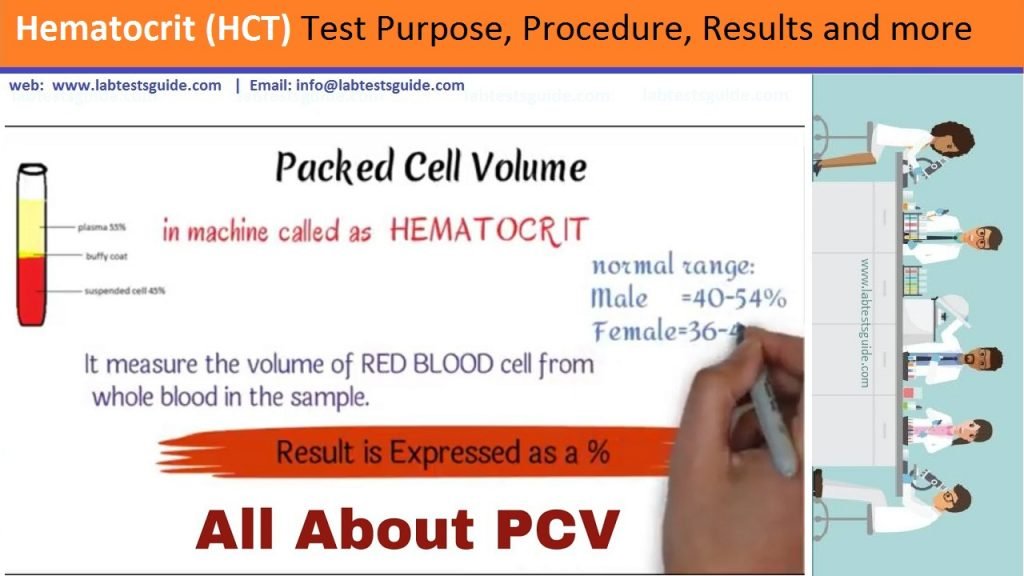
Hematocrit is the percentage of red blood cells in the total blood volume. Red blood cells are vital to your health. Imagine them as the subway system of your blood. They transport oxygen and nutrients to various locations in your body. For you to stay healthy, your body needs to have the correct proportion of red blood cells.
Also Known as: Hct, Crit, Packed Cell Volume, PCV, H and H (Hemoglobin and Hematocrit)
Test Panel: Hemoglobin, Red Blood Cells (RBC), HCT, MCV, MCH, MCHC, Platelets Count, White Blood Cells (WBC), DLC, ESR
Method to estimate the Hct:
1. Microhematocrit tube method.
Hematocrit (PCV) is the measure of the ratio of the volume occupied by the red blood cells to the volume of whole blood. The blood sample is drawn into a capillary and centrifugated, and then the ratio can be measured and expressed as a decimal or percentage fraction.
Test Requirements:
- Whole blood from a freely flowing skin puncture or anticoagulated (EDTA or heparin) venous or arterial blood
- Glass capillary tubes with a narrow diameter
- Sealing compound (if the capillaries are not self-sealing)
- Microhaematocrit centrifuge
- Microhaematocrit reader
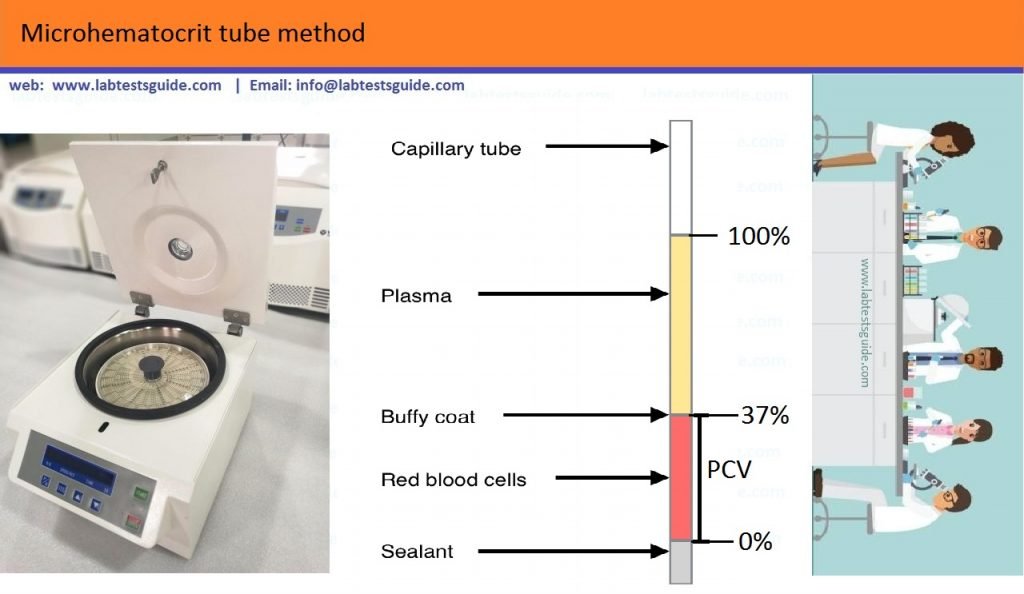
Procedure:
- Fill The Whole or anticoagulant Blood About three quarters of Tube.
- Seal the unfilled end of the capillary using a sealant material.
- Centrifuge for 5 minutes (RCF 12 000–15 000 xg).
- Immediately after centrifuging, read the PCV. First check that there has been no leakage of blood from the capillary or breakage.
To read the PCV in a hand-held microhaematocrit reader, align the base of the red cell column (above the sealant) on the 0 line and the top of the plasma column on the 100 line. Read off the PCV from scale. The reading point is the top of the red cell column, just below the buffy coat layer (consisting of WBCs and platelets).
When no reader is available: Use a ruler to measure the length of the total column of blood (top of plasma to bottom of red cell column) in mm and the length of the red cell column (base to below buffy coat layer). Calculate the PCV as follows:

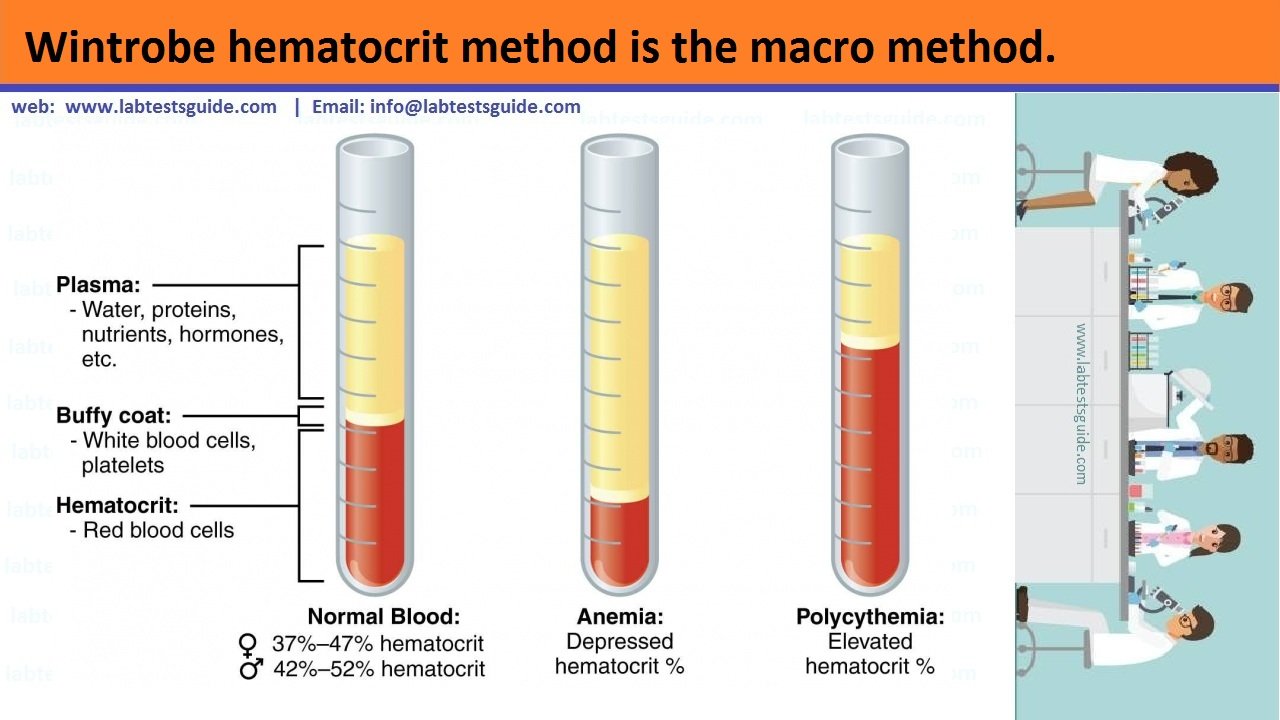
2. Wintrobe hematocrit method is the macro method.
Anticoagulated whole blood is centrifuged in a Wintrobe tube to completely pack the red cells. The volume of packed red cells is read directly from the tube. An advantage with this method is that before performing PCV, test for erythrocyte sedimentation rate can be set up.
Requirements:
- Venous blood collected in EDTA (1.5 mg EDTA for 1 ml of blood) or in double oxalate. Test should be performed within 6 hours of collection.
- Wintrobe tube: This tube is about 110 mm in length and has 100 markings, each at the interval of 1 mm. Internal diameter is 3 mm. It can hold about 3 ml of blood.
- Pasteur pipette with a rubber bulb and a sufficient length of capillary to reach the bottom of the Wintrobe tube.
- Centrifuge with a speed of 2300 g
Method:
- Mix the anticoagulated blood sample thoroughly.
- Draw the blood sample in a Pasteur pipette and introduce the pipette up to the bottom of the Wintrobe tube. Fill the tube from the bottom exactly up to the 100 mark. During filling, tip of the pipette is raised, but should remain under the rising meniscus to avoid foaming.
- Centrifuge the sample at 2300 g for 30 min (To counterbalance a second Wintrobe tube filled with blood from another patient or water should be placed in the centrifuge).
- Measure the height of the RBCs layer in mm. The obtained figure is the packed cell volume and is expressed in percentage.
Hematocrit can be expressed either as a percentage or as a fraction of the total volume of blood sample.
3. Automated method.
Use any Automated Hematology Analyzer. Some Hematology Analyzer here
- Diatron – Abacus Junior
- Mindray – BC-5800
- Nihon Kohden – Celltac Alpha MEK-6500K
- Norma Diagnostics – Icon 3
- Sysmex – XS 500i
- Beckman Coulter – MicroDiff
- Beckman Coulter – MAXM
- Beckman Coulter – HmX
- Orphee – Mythic 18
- Human Diagnostics – HumaSed Line
- Abbott Diagnostics – Cell-Dyn 3500
- Abbott Diagnostics – Cell-Dyn 3200
- Abbott Diagnostics – CELL-DYN 1800