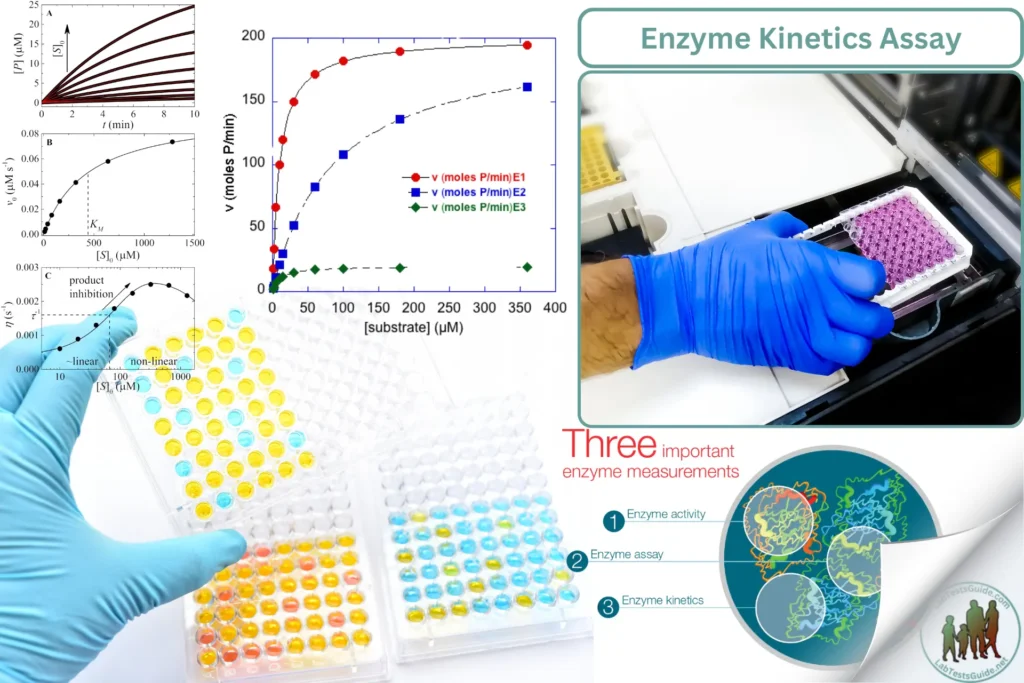
Enzyme kinetics assays are used in clinical laboratories to study the rate of enzymatic reactions, measure enzyme activity, and assess the effect of inhibitors or substrates on enzymatic activity. These assays provide valuable information for diagnosing enzyme deficiencies and monitoring treatment responses.
Methodology for Enzyme Kinetics Assay in Clinical Laboratory
Introduction:
Enzyme kinetics assays are used in clinical laboratories to study the rate of enzymatic reactions, measure enzyme activity, and assess the effect of inhibitors or substrates on enzymatic activity. These assays provide valuable information for diagnosing enzyme deficiencies and monitoring treatment responses.
Principle:
Enzyme kinetics assays measure the rate of enzymatic reactions by monitoring the change in substrate concentration over time or the production of product. The assays typically involve measuring absorbance, fluorescence, or luminescence as indicators of enzyme activity.
Specimen Requirements:
- Type of specimen: Typically purified enzymes or enzyme-containing biological samples (e.g., serum, plasma, tissue extracts)
- Volume of specimen required: Variable depending on the specific assay and enzyme concentration
- Collection method: Standard clinical procedures for sample collection (e.g., venipuncture)
- Handling and storage requirements: Maintain samples at appropriate temperatures (e.g., 2-8°C or frozen) depending on enzyme stability
- Stability of the specimen: Enzyme stability varies; handle samples according to specific guidelines and avoid repeated freeze-thaw cycles
Reagents and Materials:
- Substrate specific to the enzyme being assayed
- Cofactors or coenzymes required for the enzymatic reaction (if applicable)
- Buffer solutions (e.g., phosphate-buffered saline, Tris-HCl) to maintain pH and provide ionic strength
- Enzyme standards or controls with known activity
- Stop solution (if needed to terminate the reaction)
- Assay detection reagents (e.g., chromogenic substrates, fluorogenic substrates)
Equipment:
- Spectrophotometer, fluorometer, or luminometer depending on the detection method
- Micropipettes and tips
- Temperature-controlled incubator or water bath
- Microplate reader (for high-throughput assays)
- Assay tubes or microplates
Procedure:
Preparation:
- Prepare enzyme standards or controls with known activity to generate a standard curve.
- Prepare assay buffers and reagents according to the assay protocol. Assay Setup:
- Dispense appropriate volumes of enzyme, substrate, cofactors, and buffer into assay tubes or microplate wells.
- Start the reaction by adding the substrate to the enzyme-containing samples and standards.
- Incubate the reaction mixture at the specified temperature and time to allow the enzymatic reaction to proceed. Reaction Monitoring:
- Measure the change in absorbance, fluorescence, or luminescence over time using the appropriate detection method.
- Record readings at regular intervals to capture the initial velocity (V0) of the reaction. Data Analysis:
- Plot the reaction rate (initial velocity) versus substrate concentration for the enzyme standards to generate a standard curve.
- Calculate the enzyme activity in the samples based on the standard curve and the initial velocity of the reaction.
- Analyze the enzyme kinetics parameters such as Michaelis-Menten constant (Km), maximum velocity (Vmax), and inhibition constants (if applicable).
Quality Control:
- Include assay controls and standards in each assay run to validate the accuracy and precision of the enzyme kinetics assay.
- Verify the linearity of the standard curve and the consistency of reaction conditions.
- Monitor temperature, pH, and incubation times to ensure reproducibility of results.
Calculation and Interpretation:
- Calculate enzyme activity (units per mL or per mg protein) based on the initial velocity and standard curve.
- Interpret the enzyme kinetics parameters to understand the enzyme’s affinity for its substrate and its catalytic efficiency.
- Compare results with established reference ranges or clinical guidelines to assess enzyme function or abnormalities.
Limitations:
- Enzyme kinetics assays require careful optimization of assay conditions (temperature, pH, substrate concentration) for accurate results.
- Assay results may be influenced by sample matrix effects or interfering substances.
- Interpretation of enzyme kinetics data requires expertise in enzymology and understanding of kinetic parameters.
Safety Precautions:
- Handle enzymes and reagents according to standard biohazard and chemical safety protocols.
- Use appropriate PPE (gloves, lab coat, safety goggles) when handling potentially hazardous materials.
- Dispose of waste according to local regulations and institutional guidelines.
References:
- Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). W H Freeman.
- Principles of Enzyme Assay and Diagnostic Applications. Springer Protocols.
This format provides a structured approach to conducting enzyme kinetics assays in clinical laboratories, focusing on measuring enzyme activity and analyzing kinetic parameters to assess enzyme function and related clinical implications.
Possible References Used