The Ziehl-Neelsen (ZN) method of the Acid Fast staining technique is used to stain Mycobacterium species, including M. tuberculosis, M. ulcerans and M. leprae, and non-tuberculous mycobacteria (NTM). Detection of acid-fast bacilli (ARB) in microscopically examined acid-washed and stained smears can provide initial bacteriological evidence for the presence of mycobacteria in a clinical specimen. Smear microscopy is the fastest and easiest procedure that can be performed.
The cell wall of mycobacteria contains a high concentration of lipids, making them waxy, hydrophobic, and impervious to routine stains, such as Gram’s stain. They are also acid and alcohol resistant and are described as Acid Fast Bacilli (AFB) or Acid Alcohol Fast Bacilli (AAFB).
There are two procedures commonly used for acid-fast staining:
- Carbolfuchsin methods which include the Ziehl-Neelsen and Kinyoun methods. ( Light /bright field microscope)
- Fluorochrome procedure using auramine-O or auramine-rhodamine dyes. (Fluorescent microscope)
Objectives of the Ziehl-Neelsen Staining
- To differentiate between acid-fast bacilli and non-acid-fast bacilli.
- To stain Mycobacterium species.
Principle of the Ziehl-Neelsen Staining
- The Ziehl-Neelsen stain uses basic fuchsin and phenol compounds to stain the cell wall of Mycobacterium species.
- Mycobacterium does not bind readily to simple stains and therefore the use of heat along with carbol-fuschin and phenol allows penetration through the bacterial cell wall for visualization.
- Mycobacterium cell wall contains high lipid content made up of mycolic acid on its cell wall making it waxy, hydrophobic, and impermeable. These are ß-hydroxycarboxylic acids made up of 90 carbon atoms that define the acid-fastness of the bacteria.
- Use of Carbol-fuschin which is basic strongly binds to the negative components of the bacteria which include the mycolic acid and the lipid cell wall. addition of acid alcohol along with the application of heat forms a strong complex that can not be easily washed off with solvents.
- The acid-fast bacilli take up the red color of the primary dye, carbol-fuschin.
- While non-acid-fast bacteria easily decolorize on the addition of the acid-alcohol and take up the counterstain dye of methylene blue and appear blue
- This technique has been used in the identification of Mycobacterium tuberculosis and Mycobacterium leprae.
Reagents used in the Ziehl-Neelsen Stain
- Carbol-Fuschin (Primary dye)
- 20% sulphuric acid or acid-alcohol (Decolorizer)
- Methylene Blue dye (counterstain) or malachite green
Acid alcohol (3% hydrochloric acid in 95% ethyl alcohol)
- Ethyl alcohol- 95 ml
- Distilled water- 2 ml
- Concentrated hydrochloric acid- 3 ml
0.25% methylene blue in 1% acetic acid
- Methylene blue- 0.25g
- Distilled water- 99ml
- Acetic acid- 1ml
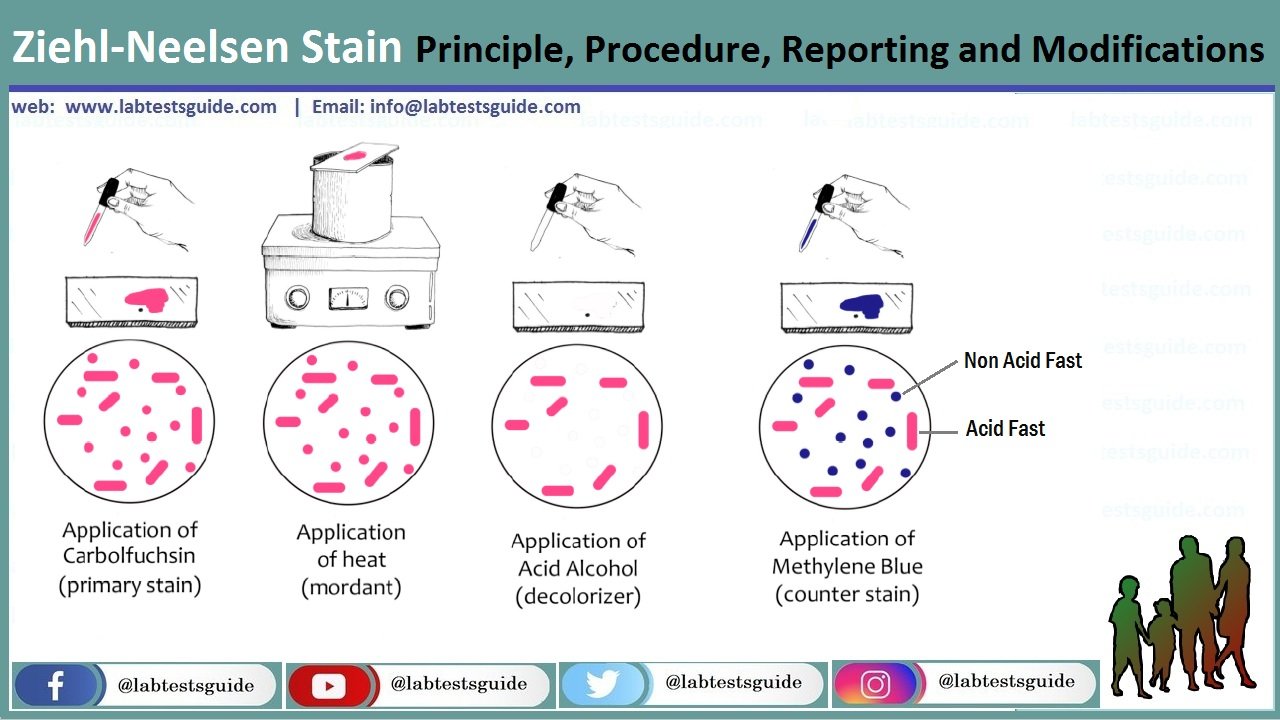
Procedure for Ziehl-Neelsen Staining

- On a clean sterile microscopic slide, make the smear of the sample culture and heat fix the smear over blue heat.
- Over the smear, pour and flood the smear with carbol fuschin and heat gently until it produces fumes.
- Allow it to stand for 5 minutes and wash it off with gently flowing tap water.
- Add 20% sulphuric acid and leave it for 1-2 minutes. Repeat this step until the smear appears pink in color.
- Wash off the acid with water.
- Flood the smear with methylene blue dye and leave it for 2-3 minutes and wash with water.
- Air dry and examine the stain under the oil immersion lens.
Results and Interpretation

- Acid-fast bacteria retain the primary dye, carbol-fuschin, and stain pink.
- Non-acid fat bacteria take up the methylene blue dye and appear blue.
- Used for examination and identification of Mycobacterium species.
- Used to differentiate between acid-fast and non-acid fast bacilli
- Used for the identification of some fungal species such as Cryptosporidium.
Results of Acid Fast Staining:
| Reagent | Acid Fast | Non-Acid Fast |
| Carbol Fuchsin with heat | Red (Hot Pink) | Red (Hot Pink) |
| Acid Alcohol | Red | Colorless |
| Methylene Blue/Malachite Green | Red | Blue/Green |
Limitations of Ziehl-Neelsen Staining
- It can only be used to identify acid-fast bacilli.
- The physical morphology of the organism is distorted.
Reporting of sputum smear
- When no AFB are seen after examining 300 fields, report the smear as ‘No AFB seen’.
- When very few AFB are seen i.e. when only 1 or 2 AFB are seen after examining 100 fields, request a further specimen to examine (Those AFB might have came from tap water (saprophytic mycobacteria), or it may be scratch of glass slide or by the use of same piece of blotting paper while drying.
- When any red bacilli are seen, report the smear as ‘AFB positive’ and give an indication of the number of bacteria present as follows (The greater the number, the more infectious the patient):
- More than 10 AFB/field at least in 20 fields: report as + + +
- 1-10 AFB/field at least in 50 fields: report as + +
- 10-99 AFB/ 100 fields: report as +
- 1-9 AFB/100 fields: report the exact number
List of Acid Fast organisms (Other than Mycobacteria)
- Nocardia spp: Partial Acid Fast
- Rhodococcus spp: Partial Acid Fast
- Legionella micdadei: Partially acid fast in tissue
- Cyst of Cryptosporidium: Acid Fast
- Cyst of Isospora: Acid Fast
Related Articles:
Possible References Used


0 Comments