- Total carbon dioxide content (TCO2) measurement is the sum of the bicarbonate, carbonic acid, and dissolved carbon dioxide (CO2) in plasma, serum or whole blood.
- In the peripheral venous blood this is used to assist in evaluating the pH status of the patient and to assist in evaluation of electrolytes.
Bicarbonate is a chemical substance that acts as a buffer and does not allow the pH of the blood to become too acidic or too basic. The kidney and lungs balance the levels of bicarbonate in the body.
If bicarbonate levels are too high or low, it might indicate a problem with those organs. Thus the bicarbonate test is helpful in detecting a number of conditions that affect the blood bicarbonate levels such as lung disease, kidney disorders and metabolic conditions.
| Test Name | BLOOD GAS ANALYSIS, ARTERIAL |
| Test Purpose | A blood gas test measures the amount of oxygen and carbon dioxide in the blood. |
| Test Preparations | No Preparation Needed |
| Test Components | pH, PCO2, Bicarbonate (HCO3), Total CO2 Contents (TCO2), Standard Bicarbonate (SBC), Base Excess, PO2, Oxygen saturation capacity, Base Excess – Extracellular fluid, Hemoglobin |
| Specimen | 2 mL arterial blood in a preheparinised (1000 IU/mL) disposable syringe and needle. Avoid air bubbles. Mix gently 10 times. Seal needle with rubber stopper. Sample to be drawn by referring doctor. Ship refrigerated. DO NOT FREEZE. Outstation samples not accepted. |
| Stability Room | 2 hrs |
| Stability Refrigerated | 24 hrs |
| Stability Frozen | NA |
| Method | ISE, Amperometry, Reflectance photometry |
Also Known as: Arterial Blood Gases, ABGs, Arterial Blood Gas Analysis, Blood Gases
Why get tested :
The bicarbonate test is performed to measure the level of bicarbonate in a person’s blood.
It may be done under the following conditions:-
- As part of a routine exam or to help evaluate a chronic or acute illness
- To detect and evaluate an electrolyte imbalance
- To monitor the effectiveness of treatment for known imbalances
- At different intervals to help monitor conditions, such as kidney disease and hypertension
- To evaluate your body’s acid-base balance (pH)
- As a part of the Electrolyte panel along with other tests like such as sodium, potassium and chloride test
When to get tested:
- When you have symptoms of a breathing problem such as shortness of breath, shortness of breath, or rapid breathing; when you are being treated for a lung disease
- when an acid-base imbalance is suspected
- Periodically when you have a condition causing acute or chronic oxygen shortage and are on oxygen therapy
- During certain surgeries to check the levels of O2 and CO2 in your blood
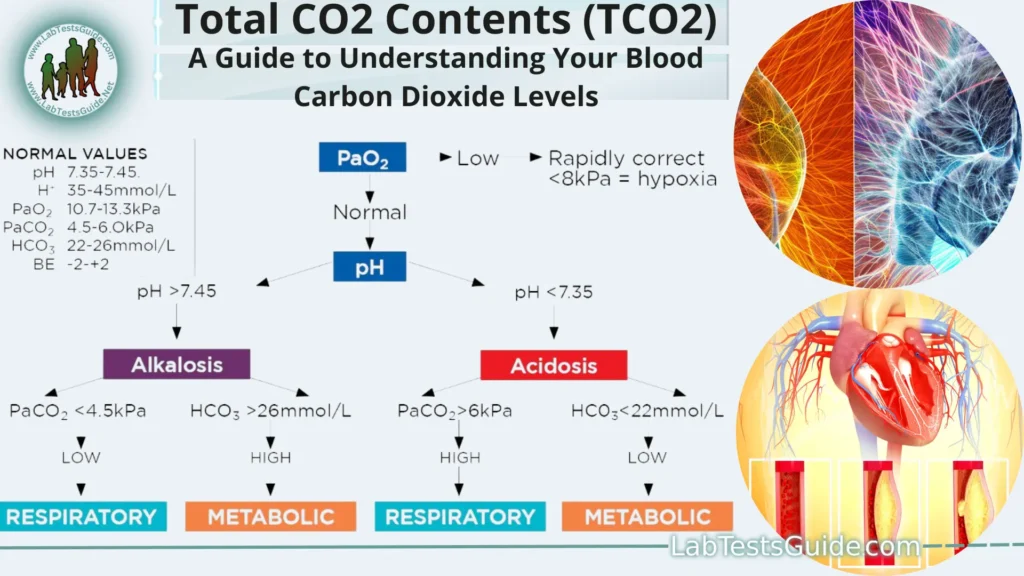
Normal Values
- 23 to 27 MEq/Lg
In the test measures:
- pH:
The pH tells you if your patient is acidotic or alkalotic. It is a measurement of the acid content or hydrogen ions [H+] in the blood. Low pH indicates a higher concentration of hydrogen ions (acidosis) while a high pH indicates a lower concentration of hydrogen ions (alkalosis). - PCO2:
The PaCO2 level is the respiratory component of the ABG. It is a measurement of carbon dioxide (CO2) in the blood and is affected by CO2 removal in the lungs. A higher PaCO2 level indicates acidosis while a lower PaCO2 level indicates alkalosis - Bicarbonate (HCO3):
level is the metabolic component of the ABG. It is a measurement of the bicarbonate content of the blood and is affected by renal production of bicarbonate. A lower HCO3 – level indicates acidosis while a higher HCO3 – level indicates alkalosis. - Total CO2 Contents (TCO2):
Total carbon dioxide content (TCO2) measurement is the sum of the bicarbonate, carbonic acid, and dissolved carbon dioxide (CO2) in plasma, serum or whole blood. In the peripheral venous blood this is used to assist in evaluating the pH status of the patient and to assist in evaluation of electrolytes. - Standard Bicarbonate (SBC):
SBC: standard bicarbonate Is the calculation of the plasma bicarbonate ion concentration after the blood has been equilibrated to a pCO2 of 40 mmHg. It is traditionally considered the reflection of a metabolic acid-base change. - Base Excess:
It is defined as the amount of acid required to restore a litre of blood to its normal pH at a PaCO2 of 40 mmHg. The base excess increases in metabolic alkalosis and decreases (or becomes more negative) in metabolic acidosis, but its utility in interpreting blood gas results is controversial. - PO2:
PO2 (partial pressure of oxygen) reflects the amount of oxygen gas dissolved in the blood. It primarily measures the effectiveness of the lungs in pulling oxygen into the blood stream from the atmosphere. Elevated pO2 levels are associated with: Increased oxygen levels in the inhaled air. - Oxygen saturation capacity:
In healthy individuals breathing room air at sea level, SaO2 is between 96% and 98%. The maximum volume of oxygen which the blood can carry when fully saturated is termed the oxygen carrying capacity, which, with a normal haemoglobin concentration, is approximately 20 mL oxygen per 100 mL blood. - Base Excess – Extracellular fluid:
It is defined as the amount of acid required to restore a litre of blood to its normal pH at a PaCO2 of 40 mmHg. The base excess increases in metabolic alkalosis and decreases (or becomes more negative) in metabolic acidosis, but its utility in interpreting blood gas results is controversial. - Hemoglobin:
Hemoglobin is the protein molecule in red blood cells that carries oxygen from the lungs to the body’s tissues and returns carbon dioxide from the tissues back to the lungs. Hemoglobin is made up of four protein molecules (globulin chains) that are connected together.
Normal Values:
| TEST | UNIT | NORMAL VALUES |
|---|---|---|
| pH | 7.35 – 7.45 | |
| PCO2 | mmHg | 35.00 – 45.00 |
| Bicarbonate (HCO3) | mEq/L | 21.00 – 28.00 |
| Total CO2 Contents (TCO2) | mEq/L | 23.00 – 27.00 |
| Standard Bicarbonate (SBC) | mEq/L | 22.00 – 26.00 |
| Base Excess | mEq/L | -2.00 – 3.00 |
| PO2 | mmHg | 83.00 – 108.00 |
| Oxygen saturation capacity | % | 95.00 – 98.00 |
| Base Excess – Extracellular fluid | mEq/L | <0.02 |
| Hemoglobin | g/dL | 13.00 – 18.00 |
Home | Blog | About Us | Contact Us | Disclaimer





3 Comments