Anti-tTG IgA Test Purpose, Procedure, Principle, Result Interpretation, Report Formate and Clinical Signification
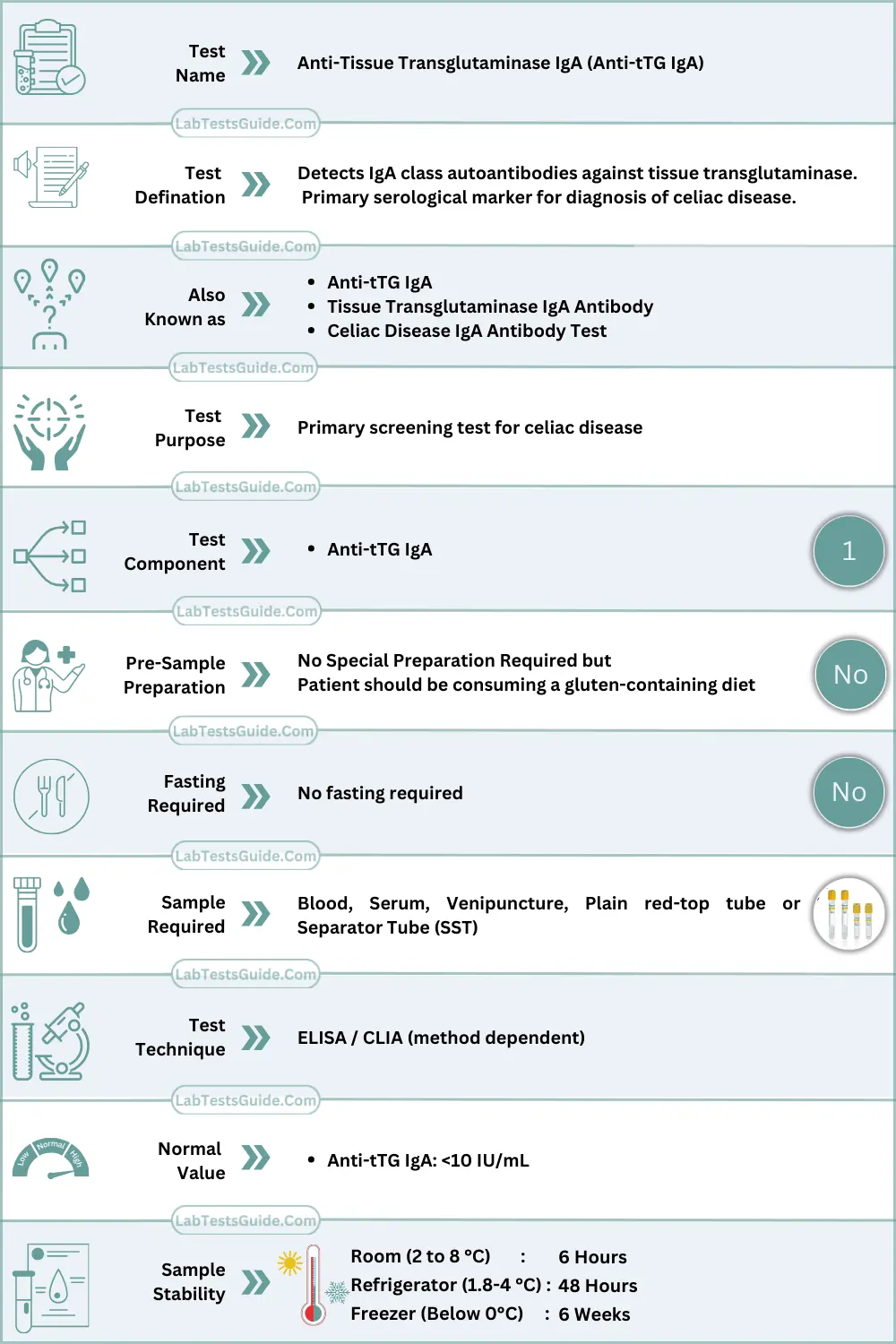
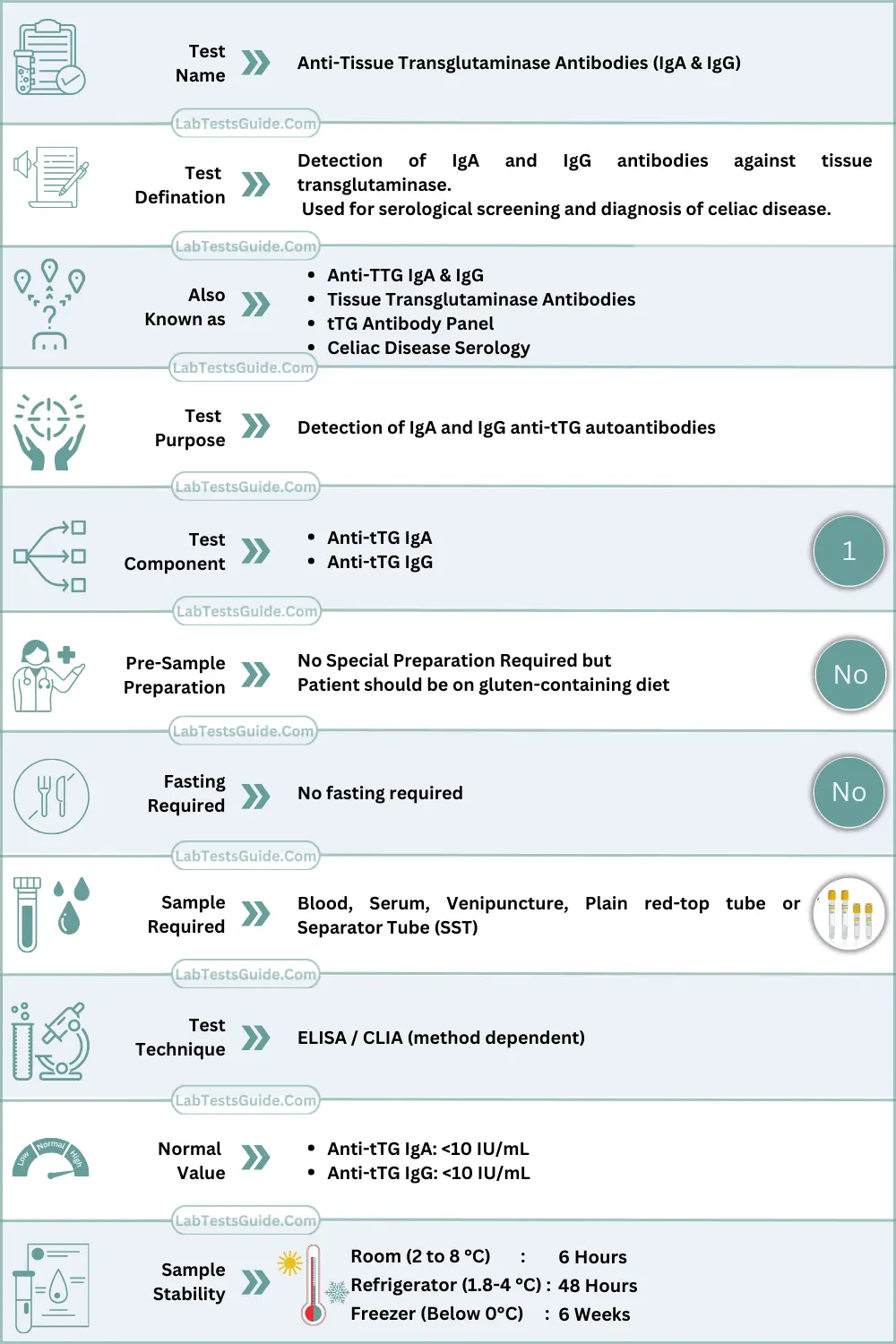
The Anti-tTG IgA assay is a class-specific immunological test designed to detect IgA autoantibodies directed against tissue transglutaminase (tTG), a calcium-dependent enzyme involved in post-translational protein modification. The test serves as the primary serological marker for immune-mediated gluten-related enteropathies and is widely used in laboratory autoimmune screening algorithms.
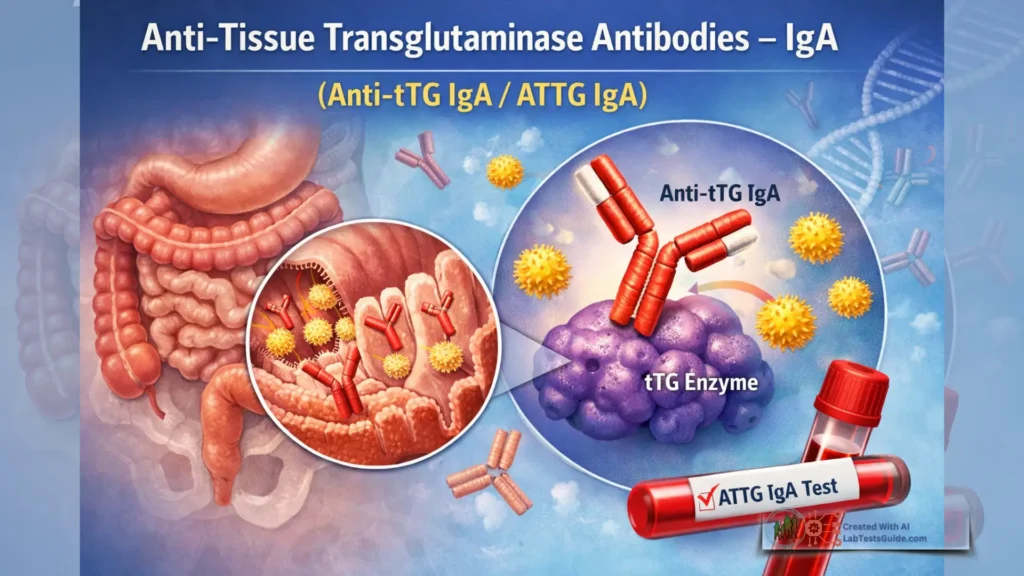
Anti TTG Test Types:
- Anti-TTG IgA Test:
- This test measures IgA (immunoglobulin A) antibodies against tissue transglutaminase.
- IgA antibodies are commonly used because they are found in the mucous membranes of the gastrointestinal tract, which is where celiac disease primarily affects.
- It is the primary form of the Anti-TTG antibody test and is considered highly specific for celiac disease diagnosis.
- Results are typically reported in units such as U/mL (units per milliliter) or IU/mL (international units per milliliter).
- Anti-TTG IgG Test:
- In some cases, individuals with celiac disease may have IgA deficiency, which can lead to false-negative results on the Anti-TTG IgA test.
- In such cases, the Anti-TTG IgG test is used to detect IgG antibodies against tissue transglutaminase.
- This test is often used as an alternative when IgA deficiency is suspected or confirmed.
- Results are also reported in units such as U/mL or IU/mL.
Principle of the Test
The ATTG IgA assay is based on a solid-phase antigen–antibody immunological reaction.
ELISA Principle
- Microtiter wells are coated with recombinant human tissue transglutaminase antigen
- IgA antibodies in patient serum bind specifically to immobilized tTG
- An enzyme-labeled anti-human IgA conjugate binds to the antigen–antibody complex
- Addition of chromogenic substrate (TMB) results in color formation
- Reaction is stopped using acid stop solution
- Color intensity is proportional to IgA antibody concentration
- Absorbance measured at 450 nm (reference 620–630 nm)
Method Type:
- Immunoassay
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chemiluminescent Immunoassay (CLIA)
- Fluorescence Enzyme Immunoassay (FEIA)
Clinical Significance (Technical)
Elevated ATTG IgA
- Indicates loss of immune tolerance to tissue transglutaminase
- Reflects IgA-mediated autoimmune mucosal injury
- Antibody titers correlate with:
- Degree of intestinal villous atrophy
- Severity of immune-mediated inflammation
- High specificity makes it the preferred first-line serological assay
Low or Undetectable ATTG IgA
- Absence of detectable IgA-mediated immune response
- May be seen in:
- Early disease
- Treated or inactive disease
- Selective IgA deficiency
- Requires correlation with total serum IgA
Specimen Requirements
| Parameter | Requirement |
|---|---|
| Specimen Type | Serum |
| Acceptable Specimen | Plasma (method-dependent) |
| Preferred Tube | Red top / SST |
| Minimum Volume | 0.5 mL serum |
| Additives | None (serum preferred) |
| Stability | 48 hours at 2–8 °C |
| Long-term Storage | −20 °C (≤3 months) |
| Transport | Refrigerated (cold chain) |
| Rejection Criteria | Hemolysis, lipemia, icterus, incorrect tube, delayed separation, contamination |
Patient Preparation (Technical)
- No fasting required
- Sample collection prior to initiation of immunosuppressive therapy is preferred
- Gluten exposure influences antibody titers
- Recent blood transfusion may interfere
- Avoid repeated freeze–thaw cycles
Reagents & Materials Required
Reagents
- Recombinant human tTG-coated microtiter wells
- Anti-human IgA enzyme conjugate (HRP-labeled)
- TMB chromogenic substrate
- Stop solution (0.5–1N sulfuric acid)
- Wash buffer (phosphate-buffered saline with surfactant)
Calibrators
- Multi-level IgA Anti-tTG calibrators traceable to manufacturer reference standards
Controls
- Negative (normal) control
- Low positive control
- High positive (pathological) control
Equipment
- Centrifuge
- ELISA washer
- Microplate reader
- Automated immunoassay analyzer (if applicable)
- Micropipettes with disposable tips
- Incubator (37 °C)
- Timer
Required Wavelength
- Primary: 450 nm
- Reference: 620–630 nm
Reagent Storage
- Store at 2–8 °C
- Do not freeze conjugates or coated plates
- Protect TMB from light
Test Procedure (Step-by-Step)
A. Manual ELISA Method
- Allow reagents and samples to reach room temperature
- Add 100 µL calibrators, controls, and samples to designated wells
- Incubate at 37 °C for 30 minutes
- Wash wells 3–5 times with wash buffer
- Add 100 µL anti-IgA enzyme conjugate
- Incubate at 37 °C for 30 minutes
- Wash thoroughly
- Add 100 µL TMB substrate
- Incubate 10–15 minutes in the dark
- Add 100 µL stop solution
- Measure absorbance at 450 nm
B. Automated Method
- Load samples, reagents, calibrators, and controls
- Program assay protocol as per analyzer instructions
- Calibration frequency: with new reagent lot or QC failure
- Analyzer performs incubation, washing, detection
- On-board reagent stability: typically 7–14 days (method-dependent)
Calculation Formula
Manual ELISA Calculation
ATTG IgA (IU/mL)=Calibrator AbsorbanceSample Absorbance×Calibrator Value
Units: IU/mL
Worked Example:
Sample OD = 1.10
Calibrator OD = 0.88
Calibrator = 10 IU/mL
Result = 12.5 IU/mL
Reference Ranges
| Population | Reference Range (IU/mL) or Cut off |
|---|---|
| Adult Male | <10 |
| Adult Female | <10 |
| Children | <10 |
| Pregnancy | <10 |
| Method-specific | As per manufacturer |
Reference ranges must be validated by each laboratory.
Interpretation (Technical / Professional)
High Levels
- IgA-mediated autoimmune response against tTG
- Active immune-mediated mucosal injury
- Correlates with:
- Anti-endomysial antibodies
- Elevated inflammatory markers
- Malabsorption-associated biochemical abnormalities
Low Levels
- Absence of IgA autoantibody response
- Possible selective IgA deficiency
- Early or treated disease state
Interfering Factors / Sources of Error
- Hemolysis → false elevation
- Lipemia → optical interference
- Icterus / high bilirubin
- Immunosuppressive drugs → decreased titers
- Delayed serum separation
- Sample contamination
- Incorrect anticoagulant
- Temperature variation
- Improper mixing
- Instrument drift
Quality Control Requirements
- QC Levels: Negative, Low Positive, High Positive
- Frequency: Daily or per analytical run
- Acceptable ranges: Manufacturer-defined
- Apply Westgard rules (1-2s, 2-2s, R-4s)
- Maintain Levey–Jennings charts
- Corrective actions: repeat QC, recalibrate, replace reagents
Calibration Requirements
- Multi-level calibration
- Perform with:
- New reagent lot
- Analyzer maintenance
- QC failure
- Linearity: typically up to ~200 IU/mL
- Recalibrate if drift or non-linearity observed
Instrument Maintenance Notes
- Daily: Probe wash, system rinse
- Weekly: Optical inspection, wash system cleaning
- Monthly: Temperature verification, carryover assessment
🧠 AI-Powered Test Result Analysis:
Understand your Anti-Tissue Transglutaminase Antibodies – IgA (Anti-tTG IgA / ATTG IgA) Test Results
AI-powered Lab Test Results Meaning tool 🤖
📥 Download Anti TTG IgA Test Report Format
Get the demo report format for Electrolytes Panel in your preferred format. These templates are fully editable and professional.
How to Download ?
| File Description | Format |
|---|---|
Anti-tTG IgA Test Report Format (Image) | .PNG ⬇️ |
Anti-tTG IgA Test Report Format (MS Word) | .DOCX ⬇️ |
Anti-tTG IgA Test Format (MS Excel) | .XLSX ⬇️ |
Anti-tTG IgA Test Format (PDF) | .PDF ⬇️ |
Anti-tTG IgG & IgA Test Report Format (Image) | .PNG ⬇️ |
Anti-tTG IgG & IgA Test Report Format (MS Word) | .DOCX ⬇️ |
Anti-tTG IgG & IgA Test Format (MS Excel) | .XLSX ⬇️ |
Anti-tTG IgG & IgA Test Format (PDF) | .PDF ⬇️ |
Critical (Panic) Values
- 100 IU/mL (method-dependent)
Follow institutional policy for notification.
Troubleshooting Guide
| Problem | Cause | Solution |
|---|---|---|
| Low absorbance | Expired conjugate | Replace reagent |
| High blank | Contaminated substrate | Prepare fresh substrate |
| Drift | Temperature instability | Re-stabilize analyzer |
| Reagent deterioration | Improper storage | Use new reagents |
| Sample clot | Poor centrifugation | Re-collect sample |
| Calibration failure | Lot mismatch | Recalibrate |
| QC out of range | System or reagent error | Repeat QC and recalibrate |
Safety Precautions
- Use PPE (gloves, lab coat, eye protection)
- Treat all specimens as biohazardous
- Dispose waste per biosafety protocols
- Follow MSDS for reagents
- Avoid aerosol generation and cross-contamination
Test Limitations
- False negatives in selective IgA deficiency
- Cross-reactivity with other autoimmune antibodies
- Non-specific reactions at very high titers
- Method-dependent sensitivity and specificity
- Not diagnostic as a standalone test
Notes for Lab Students
- ATTG IgA is the primary screening assay
- Always verify QC before reporting
- Strict incubation timing is critical
- Always correlate with total IgA levels
FAQs:
What immunoglobulin class is detected in ATTG IgA testing?
IgA
Primary wavelength used in ELISA detection?
450 nm
Major cause of false-negative ATTG IgA results?
Selective IgA deficiency
Which enzyme is targeted by ATTG antibodies?
Tissue transglutaminase
Which Westgard rule detects random error?
R-4s rule
References (Technical)
- CLSI Immunoassay Guidelines
- WHO Laboratory Quality Manuals
- IFCC Autoimmune Diagnostics Documentation
- Manufacturer ELISA / CLIA Package Inserts
- Peer-reviewed Immunology Journals