Safety and Ergonomics in the Histopathology Laboratory: Risk Management
Histopathology laboratories are essential for diagnosing diseases and advancing medical research. However, they are also environments where numerous workplace hazards exist, ranging from chemical exposures to biological risks. Over the years, many countries have implemented regulations to improve safety in these laboratories. While these regulations vary, the underlying goal is universal: to protect the health and safety of laboratory personnel and the environment. This article outlines a comprehensive risk management plan tailored to histopathology laboratories, covering hazard identification, risk minimization, implementation strategies, and ongoing safety practices.

Risk Management in the Histopathology Laboratory
Risk management in histopathology laboratories is not just about personal safety; it also encompasses environmental health and safety. Despite significant improvements in workplace conditions, laboratories remain potential sources of environmental pollution. The goal of this article is to provide a globally applicable risk management framework, addressing the unique hazards of histopathology laboratories.
Identify and Evaluate Hazards
The first step in risk management is identifying and evaluating hazards in the workplace. This process can be daunting, especially in laboratories with outdated or poorly labeled chemicals. Key steps include:
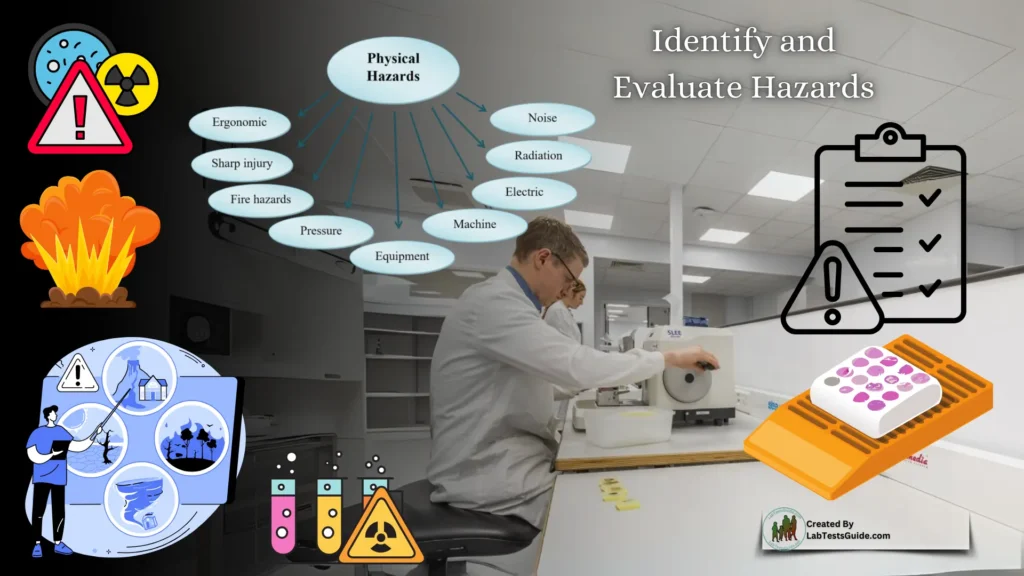
- Chemical Inventory: Create a detailed inventory of all chemicals, including their location and associated procedures.
- Hazard Classification: Identify hazards beyond chemicals, such as electrical, mechanical, and biological risks.
- Safety Data Sheets (SDS): Maintain up-to-date SDS for all chemicals, ensuring they are accessible to all employees.
- Risk Evaluation: Assess the severity of each hazard, considering factors like volume, frequency of use, and potential for spillage or exposure.
For example, the risks associated with a large formalin spill in a laboratory differ significantly from those of a small spill in a clinical setting. Always evaluate risks proportionally to the scale of operations.
Plan to Minimize Risk
Once hazards are identified and evaluated, the next step is to develop a plan to minimize risks. This involves:
- Work Practice Controls: Eliminate, reduce, or recycle hazardous materials wherever possible.
- Engineering Controls: Implement ventilation systems, fire protection devices, and other facility modifications.
- Personal Protective Equipment (PPE): Use PPE as a last resort when other controls are insufficient.
Prioritize eliminating hazards entirely. For instance, many laboratories have replaced hazardous chemicals like benzene and xylene with safer alternatives. If elimination is not feasible, consider reducing usage or recycling materials.
Implement the Plan
A risk management plan is only effective if implemented. Prioritize changes based on their impact and feasibility. Start with easy-to-implement measures that yield immediate benefits, such as cost savings or reduced exposure risks. For example, switching to formalin substitutes may have higher upfront costs but can lead to long-term savings in monitoring and disposal.
Design Standard Operating Procedures (SOPs)
SOPs are critical for ensuring consistent and safe handling of hazardous materials. Key components of SOPs include:
- Personal Hygiene Practices: Define protocols for handwashing, glove use, and other hygiene measures.
- Control Measures: Specify when and how to use PPE, fume hoods, and other safety equipment.
- Spill Procedures: Outline steps for handling spills, including when to call HazMat responders.
- Training and Medical Support: Include provisions for employee training, medical consultations, and examinations.
Establish a qualified safety officer or committee to oversee the development and implementation of SOPs.
Train Personnel
Safety training is mandatory in many countries and is essential for reducing risks. Training should cover:
- General Safety Practices: Basic laboratory safety protocols.
- Specific Hazards: Handling carcinogens, formaldehyde, and other high-risk substances.
- Documentation: Maintain records of training, including employee signatures and dates.
Annual retraining is recommended, and new employees should receive training before starting work.
Periodic Reviews
Regularly review and update SOPs, risk assessments, and training programs. Address new hazards introduced by changes in laboratory operations or workloads. Ensure all documents are dated and revised as needed.
Record Keeping
Maintain detailed records of:
- Regulatory Compliance: Documentation of adherence to safety regulations.
- Risk Assessments: Records of hazard evaluations and mitigation plans.
- Employee Training: Certifications and training logs.
- Exposure Monitoring: Data on occupational exposure levels.
- Waste Disposal: Documentation of hazardous waste management practices.
Records should be kept for at least 30 years after an employee’s tenure, as required by many regulatory agencies.
Occupational Exposure Limits
Occupational exposure limits (OELs) define the maximum allowable concentration of hazardous substances in the workplace. Key terms include:
- TWA (Time-Weighted Average): Average exposure over an 8-hour workday.
- STEL (Short-Term Exposure Limit): Maximum exposure during any 15-minute period.
- CL (Ceiling Limit): Instantaneous exposure limit.
- IDLH (Immediately Dangerous to Life or Health): Concentrations that pose immediate risks.
Monitor employee exposure levels rather than ambient workplace concentrations to ensure compliance with OELs.
Biological Exposure Indices (BEIs)
BEIs measure the concentration of hazardous substances in biological samples (e.g., urine, blood) to assess exposure. For example, xylene exposure can be detected through methylhippuric acid levels in urine. BEIs serve as indicators of significant exposure but are not diagnostic tools.
Types of Hazards
Histopathology laboratories face a variety of hazards, including:

- Biohazards: Infectious agents or contaminated materials.
- Irritants: Chemicals causing reversible inflammation.
- Corrosives: Substances that destroy tissue or materials.
- Sensitizers: Chemicals causing allergic reactions.
- Carcinogens: Substances known to cause cancer.
- Toxic Materials: Chemicals causing death or severe harm at specific concentrations.
- Combustibles and Flammables: Materials with low flash points posing fire risks.
- Explosives and Oxidizers: Substances that can explode or promote combustion.
Conclusion
Effective risk management in histopathology laboratories requires a proactive approach to identifying, evaluating, and mitigating hazards. By implementing robust safety protocols, training personnel, and maintaining thorough records, laboratories can create a safer and more sustainable working environment. While regulations vary by country, the principles outlined in this article provide a universal framework for managing risks in histopathology laboratories. Prioritizing safety not only protects personnel but also enhances the overall quality and reliability of laboratory operations.