Mueller Hinton Agar (MHA) is a microbiological growth medium that is commonly used for antibiotic susceptibility testing, specifically disk diffusion tests. It is also used to isolate and maintain Neisseria and Moraxella species.
Introduction to Mueller Hinton Agar (MHA):
Mueller Hinton Agar (MHA) is a solid culture medium used in microbiology laboratories for the cultivation and antimicrobial susceptibility testing of bacteria. It was first developed by John Howard Mueller and Jane Hinton in the 1940s and has since become a widely utilized medium in clinical and research settings.
Development and History:
Mueller Hinton Agar (MHA) was developed in the 1940s by John Howard Mueller and Jane Hinton at the National Institutes of Health (NIH) in the United States. They created MHA to provide a standardized medium for microbiological research and antimicrobial susceptibility testing. The formulation included beef infusion, casein hydrolysate, starch, agar, calcium carbonate, and pH indicators. MHA quickly gained recognition for its reliability and consistency, becoming widely adopted in laboratories worldwide. Over time, minor modifications have been made, but the basic principles established by Mueller and Hinton remain unchanged. MHA continues to be a fundamental medium in antimicrobial susceptibility testing and research.
Usage and Applications of Mueller Hinton Agar:
- Antimicrobial Susceptibility Testing: Mueller Hinton Agar is used to determine the sensitivity or resistance of bacteria to antibiotics.
- Clinical Microbiology: It is employed in clinical laboratories to guide antibiotic treatment decisions based on bacterial susceptibility.
- Research Studies: Mueller Hinton Agar is used in research to study antimicrobial agents, resistance mechanisms, and test novel compounds.
- Quality Control: It serves as a tool to ensure the accuracy and reliability of antimicrobial susceptibility testing.
- Education and Training: Mueller Hinton Agar is used in educational programs to teach students the principles of susceptibility testing.
- Surveillance of Antibiotic Resistance: It helps monitor and track patterns of antibiotic resistance in bacterial populations.
- Veterinary Medicine: Mueller Hinton Agar is used in veterinary settings to test bacterial susceptibility and guide treatment decisions.
- Pharmaceutical Industry: It is used during the development and testing of new antibiotics to assess their efficacy against bacterial pathogens.
Composition of MHA:
Table with a brief description of the composition of MHA:
| Ingredient | Quantity | Description |
|---|---|---|
| Beef extract | 2.0 g | Provides nitrogen, vitamins, and other essential nutrients. |
| Casein hydrolysate | 17.5 g | Provides amino acids, peptides, and other essential nutrients. |
| Starch | 1.5 g | Provides energy and helps to solidify the medium. |
| Agar | 17.0 g | Provides a solid substrate for bacterial growth. |
| pH | 7.3 ± 0.1 | The pH is adjusted to neutral to ensure that the medium is suitable for the growth of a wide variety of bacteria. |
MHA is a non-selective medium, meaning that it will support the growth of a wide variety of bacteria. It is also a non-supplemented medium, meaning that it does not contain any additional nutrients or growth factors. This makes it a good choice for antibiotic susceptibility testing, as the results are not affected by the presence of other substances in the medium.thumb_upthumb_downuploadGoogle itmore_vert
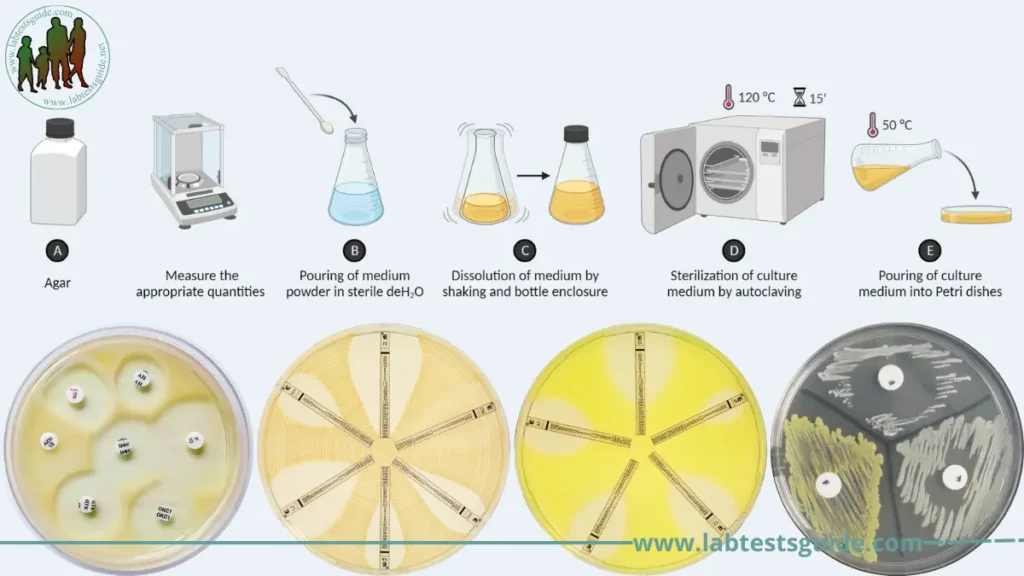
Preparation of MHA:
The preparation of Mueller Hinton Agar (MHA) involves several steps. Here’s a general outline of the process:
- Weigh the ingredients: Measure the appropriate quantities (38grams) of beef infusion or beef extract, acid hydrolysate of casein, starch, agar, calcium carbonate, and any pH indicators as per the desired batch size.
- Dissolve the ingredients: In a suitable container, add 1000 ml distilled water and gradually add the weighed ingredients while stirring continuously. Heat with frequent agitation and boil for one minute to completely dissolve the medium.
- Adjust pH (if necessary): If required, adjust the pH of the medium to the desired level using either acidic or alkaline solutions. Typically, the pH of MHA is adjusted to 7.2-7.4.
- Sterilize the medium: Autoclave the MHA mixture at a temperature of around 121°C (250°F) under pressure for approximately 15 minutes to ensure complete sterilization.
- Pouring plates: After autoclaving, allow the medium to cool down to approximately 50-55°C (122-131°F) while avoiding excessive solidification. Pour the sterilized MHA into sterile Petri dishes to a desired depth (usually around 4-6 mm) to form agar plates.
- Allow the plates to solidify: Leave the poured plates undisturbed in a horizontal position until the agar solidifies completely. It typically takes around 15-20 minutes for the plates to solidify at room temperature.
- Storage: Once the plates have solidified, they can be stored in a cool, dry place or refrigerated (2-8 ºC) until ready for use. Ensure that the plates are properly labeled with the date of preparation and any relevant information.
It’s important to note that specific protocols and variations may exist depending on the laboratory’s standard operating procedures and the specific requirements of antimicrobial susceptibility testing. Therefore, it is recommended to consult the laboratory’s guidelines or established protocols for the precise preparation and storage of Mueller Hinton Agar.
Modifications of Muller Hinton agar (MHA):
- Mueller Hinton agar medium supplemented with 5% sheep blood is recommended for determining the antimicrobial susceptibility of
- Streptococcus pneumoniae
- Neisseria meningitidis
- Haemophilus test medium (HTM) is the preferred medium for the antimicrobial susceptibility testing of H. influenzae using the modified Kirby Bauer disk diffusion method. HTM medium consists of the following ingredients: thymidine-free MHA supplemented with 15 μg/ml NAD, 15 μg/ml bovine hemin, and 5 mg/ml yeast extract.
Antimicrobial Susceptibility Testing:
- Isolate Identification: Antimicrobial susceptibility testing (AST) determines the susceptibility or resistance of bacteria or fungi to antimicrobial agents.
- Inoculum Preparation: It helps guide the selection of appropriate antimicrobial therapy for infections.
- AST Method Selection: AST methods include the Kirby-Bauer disk diffusion, broth microdilution, E-test, and automated systems.
- Mueller Hinton Agar (MHA): Mueller Hinton Agar (MHA) is commonly used as the medium for AST.
- Application of Antimicrobial Agents: Antimicrobial agents are applied to the agar surface, and the plates are incubated under controlled conditions.
- Measurement and Interpretation: Zones of inhibition around the antimicrobial discs are measured and compared to interpretive criteria to determine susceptibility.
- Reporting: AST results are reported to guide clinicians in choosing effective antimicrobial treatment.
Interpretation of Results:
Here are the steps on how to interpret the results of antibiotic susceptibility testing using Mueller Hinton Agar (MHA):
- Look for the zone of inhibition around each antibiotic disk. The zone of inhibition is the clear area around the disk where the bacteria did not grow.
- Compare the zone of inhibition to the interpretive criteria for the antibiotic. The interpretive criteria are tables that list the minimum inhibitory concentrations (MICs) for different bacteria and antibiotics.
- If the zone of inhibition is equal to or greater than the MIC for the antibiotic, then the bacteria is susceptible to the antibiotic. If the zone of inhibition is less than the MIC for the antibiotic, then the bacteria is resistant to the antibiotic.
Here are some examples of how to interpret the results of antibiotic susceptibility testing using MHA:
- If the zone of inhibition around a penicillin disk is 15 mm, then the bacteria is susceptible to penicillin.
- If the zone of inhibition around a tetracycline disk is 5 mm, then the bacteria is resistant to tetracycline.
- If the zone of inhibition around a vancomycin disk is 0 mm, then the bacteria is highly resistant to vancomycin.
Interpretation of antimicrobial susceptibility testing (AST) results involves analyzing the zones of inhibition observed around antimicrobial discs on Mueller Hinton Agar (MHA) plates. Here’s a list with brief descriptions of the interpretation of results:
- Susceptible (S):
- Zone of inhibition meets or exceeds predefined breakpoints for susceptibility.
- Indicates the tested organism is likely to respond to the antimicrobial agent.
- The antimicrobial agent is considered effective in treating infections caused by the organism.
- Resistant (R):
- Zone of inhibition falls below predefined breakpoints for resistance.
- Suggests the tested organism is not susceptible to the antimicrobial agent.
- The antimicrobial agent is unlikely to be effective in treating infections caused by the organism.
- Intermediate (I):
- Zone of inhibition falls within an intermediate range.
- No clear indication of susceptibility or resistance.
- Clinical outcome may vary based on other factors.
- Individualized decision-making is required considering the infection site, host factors, and other considerations.
It is crucial to refer to specific interpretive criteria provided by organizations like CLSI or EUCAST for accurate interpretation. Clinical judgment, patient history, and other laboratory findings should be considered alongside AST results to guide appropriate antimicrobial therapy.
Advantages, Disadvantages and Limitations:
Advantages of MHA:
- Standardized medium: MHA is a widely used and standardized medium for antimicrobial susceptibility testing, ensuring consistent and reliable results.
- Broad spectrum: MHA supports the growth of a wide range of bacteria, making it suitable for testing the susceptibility of different bacterial species to various antimicrobial agents.
- Compatibility: MHA is compatible with multiple AST methods, including disk diffusion, broth microdilution, and E-test, providing flexibility in testing approaches.
- Readability: MHA plates offer good clarity, allowing for easy visualization and accurate measurement of the zone of inhibition around antimicrobial discs.
- Practicality: MHA preparation is relatively simple and cost-effective compared to specialized media, making it a practical choice for routine antimicrobial susceptibility testing.
- Wide availability: MHA is commercially available and widely used in clinical and research settings, ensuring accessibility for laboratories worldwide.
- Consistency: Due to its standard formulation, MHA provides consistent results, allowing for comparability across different laboratories and studies.
- Well-established interpretive criteria: There are established interpretive criteria and breakpoints available for MHA, facilitating the interpretation of results and aiding in guiding antibiotic therapy decisions.
Limitations of MHA:
- Fastidious organisms: MHA may not support the growth of certain fastidious bacteria that have specific nutritional requirements, requiring the use of specialized media or modifications.
- Clinical relevance: While MHA provides valuable information on antimicrobial susceptibility in vitro, the correlation with clinical outcomes may not always be perfect due to factors such as host immune response and drug penetration into specific body sites.
- Interpretation challenges: Interpreting zone sizes on MHA plates requires expertise and adherence to predefined breakpoints, as the zone diameter can be influenced by factors such as inoculum density, pH variations, or growth conditions.
- Resistance mechanism detection: MHA alone cannot detect specific resistance mechanisms, such as enzymatic or genetic resistance determinants. Additional tests are often required to determine the underlying mechanisms of resistance.
- Fungal susceptibility testing: While MHA can be used for some fungal organisms, it may not provide optimal conditions for all fungal species, limiting its use in fungal susceptibility testing.
- Limitations in specialized testing: MHA may not be suitable for certain specialized testing, such as detecting low-level antibiotic resistance or evaluating specific antimicrobial combinations.
- Varied performance with specific antimicrobials: MHA may exhibit variations in performance with certain antimicrobial agents, potentially affecting the accuracy of susceptibility testing for those specific agents.
Disadvantages of MHA:
- Limited support for fastidious organisms: MHA may not provide the necessary growth conditions for certain fastidious bacteria that have specific nutritional requirements, limiting its utility in susceptibility testing for those organisms.
- In vitro versus in vivo correlation: The results obtained from MHA may not always directly correlate with clinical outcomes, as factors such as host immune response, tissue penetration, and drug interactions can impact the effectiveness of antimicrobial therapy.
- Interpretation challenges: Interpreting zone sizes on MHA plates can be subjective and influenced by various factors, including inoculum density, pH variations, and growth conditions, leading to potential discrepancies in result interpretation.
- Inability to detect specific resistance mechanisms: MHA alone cannot identify the specific resistance mechanisms responsible for microbial resistance to antimicrobial agents. Additional testing, such as molecular techniques, may be required for accurate identification of resistance mechanisms.
- Not optimized for fungal susceptibility testing: While MHA can be used for some fungal species, it is primarily designed for bacterial susceptibility testing. Other specialized media may be more suitable for accurate fungal susceptibility testing.
- Variability with certain antimicrobial agents: MHA may exhibit variability in performance with specific antimicrobial agents, which can impact the accuracy and reliability of susceptibility testing for those agents.
- Lack of sensitivity for low-level resistance: MHA may not be able to detect low-level resistance, where the zone sizes may be within the susceptible range but still indicate reduced susceptibility to the antimicrobial agent.
FAQs:
What is Mueller Hinton Agar (MHA)?
Mueller Hinton Agar (MHA) is a solid culture medium used in microbiology for antimicrobial susceptibility testing. It provides a standardized environment to evaluate the effectiveness of antimicrobial agents against bacterial pathogens.
Why is MHA preferred for antimicrobial susceptibility testing?
MHA is preferred for antimicrobial susceptibility testing due to its standardized composition, compatibility with various testing methods, and wide availability. It supports the growth of a broad range of bacteria and provides consistent and reproducible results.
How is MHA prepared?
MHA is prepared by dissolving the dehydrated MHA powder in water, sterilizing the medium, and pouring it into sterile petri dishes. The plates are allowed to solidify before use.
What are the interpretive criteria used for AST on MHA?
Interpretive criteria are predefined zone diameter breakpoints provided by organizations such as CLSI or EUCAST. These criteria guide the interpretation of zone sizes to determine whether a bacterium is susceptible, intermediate, or resistant to a specific antimicrobial agent.
Can MHA be used for fungal susceptibility testing?
While MHA is primarily designed for bacterial susceptibility testing, it can be used for some fungal species. However, there are other specialized media available that are more suitable for accurate fungal susceptibility testing.
How long should MHA plates be incubated for AST?
The incubation time for MHA plates in AST can vary depending on the bacterial species and the antimicrobial agent being tested. Typically, plates are incubated for 16-18 hours at 35-37°C, but specific guidelines should be followed.
Can MHA detect specific resistance mechanisms?
MHA alone cannot detect specific resistance mechanisms in bacteria. Additional testing, such as molecular techniques or specific assays, may be required to identify the underlying resistance mechanisms responsible for antimicrobial resistance.
What are the limitations of using MHA for susceptibility testing?
Some limitations of MHA include limited support for fastidious organisms, challenges in correlating in vitro results with clinical outcomes, subjective interpretation of zone sizes, and the inability to detect specific resistance mechanisms.
How does MHA contribute to antibiotic stewardship?
MHA, as a standardized medium for AST, plays a crucial role in antibiotic stewardship by providing valuable information on bacterial susceptibility patterns. This information helps guide clinicians in selecting appropriate antibiotics and optimizing treatment to minimize the development and spread of antibiotic resistance.
Conclusion:
In conclusion, Mueller Hinton Agar (MHA) is a widely used medium for antimicrobial susceptibility testing in microbiology. It offers several advantages, including standardization, compatibility with different testing methods, broad spectrum support, readability of zone sizes, simplicity in preparation, and cost-effectiveness. However, it also has limitations, such as limited support for fastidious organisms, challenges in correlating in vitro results with clinical outcomes, subjective interpretation of zone sizes, inability to detect specific resistance mechanisms, and suboptimal performance in fungal susceptibility testing.
Despite its limitations, MHA remains a valuable tool in guiding antibiotic therapy decisions, monitoring antibiotic resistance trends, conducting research studies, and supporting quality control in clinical microbiology laboratories. By considering MHA results in conjunction with clinical judgment, patient history, and other laboratory findings, healthcare providers can make informed decisions regarding antibiotic treatment and contribute to antibiotic stewardship efforts. Continuous research and development in the field of antimicrobial susceptibility testing will further enhance the utility and effectiveness of MHA in the future.
Home | Blog | About Us | Contact Us | Disclaimer





