Fluorescence In Situ Hybridization (FISH) is a molecular biology technique used to visualize and locate specific nucleic acid sequences within cells or tissue samples. It is a powerful tool in genetics and cytogenetics research and is commonly employed in clinical diagnostics and cancer research.
Key Points:
- Molecular Biology Technique – FISH is a molecular biology technique used to study and visualize specific DNA or RNA sequences within cells or tissues.
- DNA/RNA Probes – It employs DNA or RNA probes labeled with fluorescent markers to bind and identify target sequences.
- Complementary Base Pairing – FISH relies on the complementary base pairing between the probe and the target sequence for detection.
- Visualization – Fluorescent signals emitted by the labeled probes are visualized under a fluorescence microscope.
- Genetic Research – FISH is widely used in genetic research to map genes and study genome organization.
- Chromosomal Abnormalities – It helps detect chromosomal abnormalities like deletions, amplifications, and translocations.
- Cancer Research – FISH is essential in cancer research for identifying genetic markers associated with cancer.
- Sample Types – FISH can be applied to various sample types, including cells, tissue sections, and organisms.
- Chromosome Number – It can determine the chromosome number and ploidy levels within cells.
- Prenatal Diagnostics – FISH is used in prenatal diagnostics to detect chromosomal abnormalities in developing fetuses.
- Preimplantation Genetic Diagnosis (PGD) – It plays a role in PGD during in vitro fertilization to select healthy embryos.
- Multicolor FISH (M-FISH) – Multiple probes with different fluorescent labels can be used simultaneously for more complex analysis.
- Spectral Karyotyping (SKY) – SKY is an advanced FISH technique that visualizes all human chromosomes in a single experiment.
- FISH-ES – FISH can identify and quantify microbial species in environmental samples for microbial ecology studies.
- Microbial Communities – It helps study microbial communities in various ecosystems.
- Genome Organization – FISH has revolutionized our understanding of genome organization and structure.
- Probe Design – Successful FISH requires careful probe design to target specific sequences of interest.
- Hybridization Optimization – Optimization of hybridization conditions is crucial for accurate results.
- Specialized Equipment – FISH relies on specialized equipment like fluorescence microscopes for analysis.
- Versatile Tool – FISH is a versatile and powerful tool with applications in genetics, cytogenetics, and clinical diagnostics.
Definition and Overview:
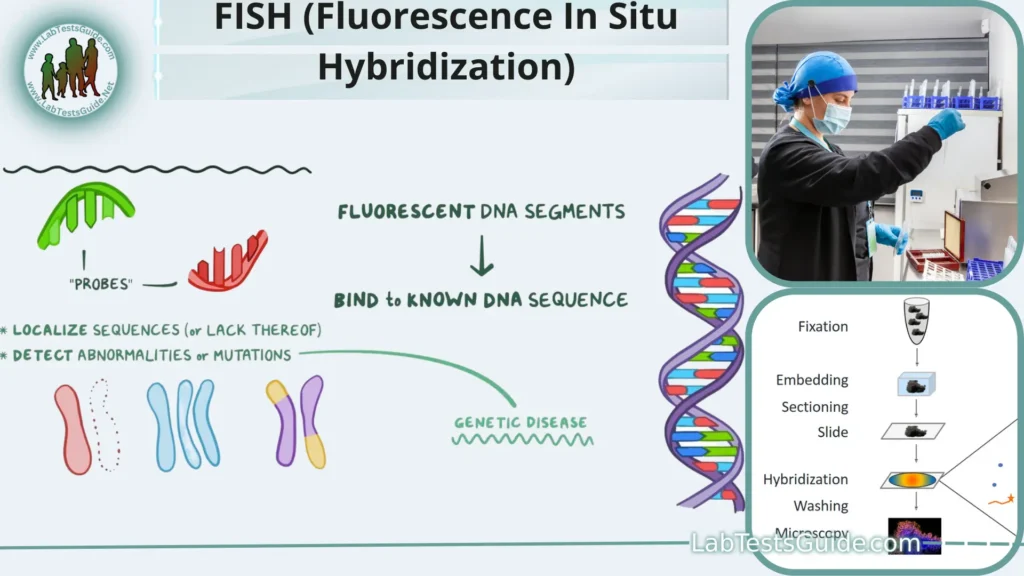
Fluorescence In Situ Hybridization (FISH) is a molecular biology technique that uses fluorescently labeled DNA or RNA probes to locate and visualize specific genetic sequences within cells or tissues. It has wide-ranging applications in genetics, cytogenetics, and clinical diagnostics. FISH revolutionizes our ability to study DNA and RNA at the cellular level by providing precise localization and identification of target sequences.
Historical Background:
Fluorescence In Situ Hybridization (FISH) has its origins in the mid-20th century. The technique evolved from earlier methods used to study chromosomal structure and organization. Here’s a brief historical overview:
- 1960s-1970s: Early studies involved using radioactively labeled DNA probes to detect complementary sequences in chromosomes. However, radioactivity posed safety concerns and limited the practicality of the method.
- 1970s-1980s: Researchers began exploring non-radioactive alternatives, leading to the development of FISH. Pioneering work by scientists like Joe Gall and Mary-Lou Pardue in the 1970s laid the foundation for the technique.
- 1980s: The term “Fluorescence In Situ Hybridization” was coined, and FISH gained popularity as a powerful tool for studying genetic material. It provided a safer and more versatile alternative to radioactive probes.
- 1986: The first publication on FISH using fluorescent probes was authored by J.W. Pinkel and D. Gray, marking a significant milestone in the technique’s development.
- 1990s-Present: FISH continued to advance rapidly, with the development of multicolor FISH (M-FISH), spectral karyotyping (SKY), and various specialized applications in genetics, cytogenetics, and clinical diagnostics.
Today, FISH remains a cornerstone of genetic research and diagnostics, enabling researchers and clinicians to explore the intricacies of DNA and RNA localization and organization within cells and tissues. Its historical evolution reflects the ongoing innovation in molecular biology and genetic analysis techniques.
Purpose of FISH:
- Localization and Mapping: FISH allows researchers to precisely locate and map the positions of genes, genetic markers, or specific DNA/RNA sequences within chromosomes. This is crucial for understanding the organization of genetic material.
- Cytogenetic Analysis: In cytogenetics, FISH is used to detect and analyze chromosomal abnormalities such as translocations, deletions, duplications, and inversions. It plays a key role in diagnosing genetic disorders.
- Cancer Research and Diagnosis: FISH is widely used in cancer research and diagnosis. It helps identify genetic alterations, such as gene amplifications or chromosomal rearrangements, associated with various cancers. This information guides treatment decisions and prognosis assessment.
- Prenatal and Preimplantation Diagnostics: FISH is employed in prenatal testing to detect chromosomal abnormalities in developing fetuses. In preimplantation genetic diagnosis (PGD), it assesses embryos for genetic abnormalities before implantation during in vitro fertilization (IVF).
- Microbiology and Environmental Studies: FISH-ES (Environmental Sample) is used to identify and quantify specific microbial species in environmental samples. It contributes to the study of microbial diversity and ecosystems.
- Gene Expression Analysis: FISH helps researchers investigate gene expression patterns by visualizing RNA molecules within cells. This is valuable for understanding how genes are regulated and how they contribute to cellular functions.
- Neurobiology and Developmental Biology: FISH is applied in neurobiology to study gene expression patterns in the brain and neuronal tissues. In developmental biology, it provides insights into gene expression during embryogenesis and tissue differentiation.
- Plant Genetics: FISH is used in plant genetics to study chromosomal organization, genetic diversity, and the identification of specific genes or markers.
- Clinical Diagnostics: FISH is routinely used in clinical laboratories for diagnosing genetic disorders, detecting cancer-related genetic alterations, and guiding treatment decisions.
- Research Tool: FISH is a versatile research tool in various biological fields, allowing scientists to investigate the genetic and molecular basis of diverse biological processes and diseases.
Principle of FISH:
The principle of FISH revolves around the specific binding of fluorescently labeled DNA or RNA probes to complementary target sequences within the genetic material of cells or tissues. Here’s how it works:
- Probe Design: Specific DNA or RNA probes are designed to be complementary to the sequences of interest. These probes are usually relatively short, typically 20-30 nucleotides in length, and are chosen to match the target DNA or RNA.
- Fluorescent Labeling: The DNA or RNA probes are labeled with fluorescent molecules, also known as fluorophores. These fluorophores emit light when exposed to specific wavelengths of light.
- Sample Preparation: The target cells or tissue sections are prepared for FISH. This may involve fixation to immobilize cellular structures and denaturation to expose the target DNA or RNA.
- Hybridization: The labeled probes are applied to the prepared sample. The probes will seek out and bind (hybridize) to their complementary sequences within the sample’s DNA or RNA.
- Washing: After hybridization, the sample is washed to remove any unbound or nonspecifically bound probes. This step reduces background fluorescence.
- Detection: The sample is examined under a fluorescence microscope equipped with appropriate filters. When exposed to specific wavelengths of light, the bound probes emit fluorescent signals at their characteristic wavelengths.
- Imaging and Analysis: A camera attached to the microscope captures images of the fluorescent signals. These images are then analyzed to determine the location, quantity, and distribution of the target sequences within the sample.
FISH Applications:
Fluorescence In Situ Hybridization (FISH) has a wide range of applications in genetics, cytogenetics, and clinical diagnostics due to its ability to visualize and locate specific DNA or RNA sequences within cells or tissues. Here are some key applications:
- Gene Mapping: FISH is used to map the location of specific genes on chromosomes, helping researchers understand the genetic basis of various traits and diseases.
- Chromosomal Abnormality Detection: It plays a crucial role in identifying chromosomal abnormalities, including deletions, duplications, translocations, and inversions, which are associated with genetic disorders.
- Cancer Research: FISH is extensively used in cancer research to detect genetic alterations such as gene amplifications, deletions, and rearrangements, aiding in the understanding of cancer development and progression.
- Prenatal Diagnostics: In prenatal testing, FISH can identify chromosomal abnormalities in developing fetuses, providing valuable information for expectant parents and healthcare providers.
- Preimplantation Genetic Diagnosis (PGD): FISH is employed during in vitro fertilization (IVF) to screen embryos for chromosomal abnormalities before implantation, reducing the risk of genetic disorders in offspring.
- Microbial Ecology: FISH-ES (Fluorescent In Situ Hybridization for Environmental Samples) is used to identify and quantify specific microbial species in environmental samples, aiding in the study of microbial communities.
- Species Identification: FISH can be used to differentiate and identify species or strains of microorganisms, making it valuable in microbiology and infectious disease research.
- Telomere Analysis: It helps researchers study telomere length and stability, which is associated with aging and cancer.
- Molecular Cytogenetics: FISH complements conventional cytogenetic techniques, providing higher resolution and precision in the analysis of chromosomes and genetic material.
- Multicolor FISH (M-FISH): M-FISH enables the simultaneous visualization of multiple DNA targets, allowing for complex chromosomal analyses and the identification of complex rearrangements.
- Spectral Karyotyping (SKY): SKY is an advanced FISH technique that can visualize all human chromosomes in a single hybridization experiment, aiding in the diagnosis of chromosomal abnormalities.
- FISH Combined with Immunohistochemistry (FISH-IHC): This technique combines FISH with immunohistochemistry to simultaneously detect genetic and protein markers, enhancing the understanding of disease mechanisms.
- Bacterial and Viral Detection: FISH is used to detect and identify specific bacterial or viral pathogens in clinical samples, contributing to infectious disease diagnosis.
- Assisted Reproduction: FISH helps assess the genetic health of embryos in assisted reproductive technologies, ensuring the selection of embryos with normal chromosomal content.
- Plant Genetics: FISH is applied in plant genetics to study chromosomal organization, genetic diversity, and the identification of specific genes or markers.
- Forensic Analysis: FISH can be employed in forensic science to identify specific DNA sequences, aiding in criminal investigations and paternity testing.
- Comparative Genomic Hybridization (CGH): FISH is used in CGH to compare the DNA copy number variations between normal and diseased cells, facilitating the identification of genetic factors in diseases.
Required Sample and its Preparation:
The choice of sample and its proper preparation are critical steps in conducting FISH experiments. Different types of samples require specific preparation procedures. Here are the key considerations for sample selection and preparation:
1. Sample Types:
- Cell Cultures: For cultured cells, samples are typically grown on coverslips or culture dishes. This method allows for controlled conditions and easy preparation.
- Tissue Sections: In clinical and histological studies, tissue sections on slides are often used. Tissues can be fresh or formalin-fixed and paraffin-embedded (FFPE).
2. Sample Selection:
- Ensure that the sample contains the target DNA or RNA sequences of interest. This might involve selecting specific cell types, regions, or tissues for analysis.
3. Sample Fixation:
- Fixation is essential to preserve cellular structures and prevent sample degradation.
- For cultured cells, fixation is usually done with formaldehyde or paraformaldehyde.
- For tissue samples, formalin fixation and paraffin embedding (FFPE) are common in clinical settings. Alternatively, fresh-frozen tissue samples can be used.
4. Slide Preparation:
- Cells or tissue sections are often mounted onto glass slides for FISH.
- In the case of FFPE samples, the tissue is thinly sliced into sections, and the sections are placed on slides.
5. Deparaffinization (if using FFPE):
- If using FFPE samples, the paraffin wax must be removed. This is done through a series of xylene and alcohol washes.
6. Denaturation:
- Denaturation is a crucial step to make the DNA or RNA accessible for probe binding. This is typically done by exposing the sample to high temperatures (e.g., 80-90°C) in a denaturation buffer.
7. Permeabilization (optional):
- In some cases, permeabilization of the cell membrane may be necessary to allow probe penetration, especially for tissue samples. This step is usually done with a detergent solution.
8. Probe Hybridization:
- After denaturation and, if needed, permeabilization, the fluorescently labeled probes are applied to the sample. These probes are typically mixed with a hybridization buffer and allowed to incubate with the sample.
9. Post-Hybridization Washes:
- Unbound probes are removed through a series of stringent washes. This step helps reduce background fluorescence.
10. Counterstaining and Mounting: – To visualize cellular structures and nuclei, counterstaining with dyes like DAPI (4′,6-diamidino-2-phenylindole) may be performed. – Finally, the sample is mounted with an anti-fade mounting medium to preserve fluorescence for imaging.
Specialized Equipment:
Fluorescence In Situ Hybridization (FISH) is a technique that requires specialized equipment to visualize and analyze the fluorescent signals emitted by labeled probes binding to target DNA or RNA sequences. Here are some of the key pieces of specialized equipment used in FISH:
- Fluorescence Microscope:
- A fluorescence microscope is the central piece of equipment for FISH.
- It has the capability to excite fluorophores with specific wavelengths of light and capture emitted fluorescence signals.
- The microscope is equipped with filters that selectively allow the passage of fluorescence emissions while blocking other wavelengths.
- High-quality optics and objectives are essential for clear and detailed imaging.
- Fluorescence Filters:
- Fluorescence microscopes are equipped with sets of excitation and emission filters specific to the fluorophores used in FISH.
- Excitation filters allow only the excitation wavelength to reach the sample.
- Emission filters allow the emitted fluorescence to pass through for detection.
- Camera and Imaging System:
- A digital camera attached to the microscope captures high-resolution images of the sample.
- Specialized imaging software controls camera settings, image acquisition, and data storage.
- The camera’s sensitivity and resolution are crucial for obtaining quality images.
- Illumination System:
- FISH requires precise and controlled illumination.
- Many fluorescence microscopes use arc lamps, lasers, or LEDs as light sources to excite fluorophores.
- Light intensity can be adjusted to optimize image quality and reduce photobleaching.
- Image Analysis Software:
- Specialized software is used for image processing, analysis, and quantification of FISH results.
- It aids in tasks such as signal counting, localization, and measurement of fluorescence intensity.
- Advanced software may also facilitate 3D image reconstruction and co-localization analysis.
- Temperature Control:
- Maintaining a stable temperature is crucial during FISH experiments, especially during the hybridization step.
- Specialized heating stages or incubation chambers can be integrated into the microscope.
- Objective Lenses:
- Microscopes are equipped with a range of objective lenses with varying magnifications and numerical apertures.
- The choice of objective lens depends on the specific FISH experiment and sample type.
- Z-Stack and 3D Imaging Accessories:
- For samples with three-dimensional structures, accessories like motorized stages and focus controllers allow the capture of Z-stack images at different focal planes.
- These images can be reconstructed to create 3D representations of the sample.
- Microscope Slide Holders and Stages:
- These components hold and position the glass slides containing the FISH samples for imaging.
- Motorized stages enable precise movement and automation of image capture.
- Quenching and Anti-fade Reagents:
- Specialized reagents may be used to reduce background fluorescence and preserve the fluorophores’ signals during imaging.
- Darkroom or Low-Light Environment (Optional):
- In some cases, a darkroom or low-light environment may be used to minimize external light interference during imaging.
Procedure of FISH:
Fluorescence In Situ Hybridization (FISH) is a multi-step molecular biology technique used to visualize and locate specific DNA or RNA sequences within cells or tissues. The procedure involves several key steps, from sample preparation to image analysis. Here’s a step-by-step overview of the FISH procedure:
1. Sample Selection and Preparation:
- Choose the appropriate sample type (e.g., cultured cells, tissue sections).
- Fix the sample to preserve cellular structures and prevent degradation.
- For clinical samples, prepare formalin-fixed and paraffin-embedded (FFPE) sections or fresh-frozen samples.
- Mount cells or tissue sections on glass slides.
2. Deparaffinization (if using FFPE samples):
- If using FFPE samples, remove paraffin wax through a series of xylene and alcohol washes.
3. Denaturation:
- Expose the sample to high temperatures (usually 80-90°C) in a denaturation buffer to make the DNA or RNA accessible for probe binding.
- This step can involve heating the sample on a hotplate or in an oven, followed by a brief cooling period.
4. Probe Hybridization:
- Prepare the fluorescently labeled DNA or RNA probes specific to the target sequences.
- Mix the probes with a hybridization buffer to create a probe solution.
- Apply the probe solution to the sample on the slide, ensuring even distribution.
- Incubate the sample at a specific temperature (typically 37-42°C) to allow the probes to bind to their complementary sequences within the sample.
5. Post-Hybridization Washes:
- Remove unbound probes through a series of stringent washes with buffers.
- These washes help reduce background fluorescence and increase signal specificity.
6. Counterstaining (optional):
- To visualize cellular structures or nuclei, apply a counterstain, often using a dye like DAPI (4′,6-diamidino-2-phenylindole).
- Counterstaining can provide context for the localization of target sequences within cells.
7. Mounting:
- Apply an anti-fade mounting medium to preserve fluorescence and protect the sample from photobleaching.
8. Microscopy and Imaging:
- Examine the sample under a fluorescence microscope equipped with appropriate filters for the fluorophores used in the probes.
- Excite the fluorescent labels with specific wavelengths of light and capture images of the emitted fluorescence.
9. Image Analysis:
- Analyze the acquired images to determine the location, quantity, and distribution of the target sequences within the sample.
- Specialized software may be used for image processing and quantification.
10. Data Interpretation: – Interpret the results based on the distribution and intensity of fluorescence signals. – Correlate the findings with the research or diagnostic objectives.
11. Documentation and Reporting: – Document the experimental details and results for publication or clinical reporting.
Probe Design:
Probe design is a critical step in the Fluorescence In Situ Hybridization (FISH) technique. The design ensures that the probes are specific to the target DNA or RNA sequences of interest, enabling accurate visualization and localization. Here’s an overview of the key considerations and steps in probe design for FISH:
1. Target Sequence Selection:
- Identify the specific DNA or RNA sequence you want to detect or visualize within the sample. This could be a gene, a chromosomal region, or a non-coding RNA.
2. Probe Length:
- Probes are typically short, usually 20-30 nucleotides in length. Longer probes may be used for larger targets.
- Ensure that the probe length is appropriate for the target sequence to enhance binding specificity.
3. Sequence Specificity:
- Design the probe to be highly complementary to the target sequence. Avoid sequences with repetitive elements or regions prone to cross-hybridization.
4. GC Content and Melting Temperature (Tm):
- Calculate the GC content and Tm of the probe to ensure stable hybridization. Probes with balanced GC content and appropriate Tm values are more likely to bind specifically to the target.
5. Avoid Secondary Structures:
- Check for potential self-complementarity or secondary structures within the probe sequence that may hinder hybridization efficiency.
6. Labeling:
- Choose a method for labeling the probe with fluorescent molecules (fluorophores). Common labels include fluorescein, Cy3, Cy5, and Alexa Fluor dyes.
- Label the probe at the 5′ or 3′ end or incorporate multiple labels along its length for increased signal intensity.
7. Quenching:
- Consider using quenchers to reduce background fluorescence. Quenchers absorb emitted light from fluorophores and prevent it from interfering with signal detection.
8. Avoid Polymorphisms:
- Check for single-nucleotide polymorphisms (SNPs) or mutations in the target sequence that might affect probe binding. Adjust the probe design accordingly.
9. Test Probes In Silico:
- Utilize bioinformatics tools and software to perform in silico analysis of probe sequences, including specificity, melting temperature, and secondary structures.
10. Validation: – Experimentally validate the probe’s specificity and efficiency through hybridization assays using control samples with known target sequences.
11. Positive and Negative Controls: – Include positive controls with known target sequences and negative controls lacking the target sequence in your FISH experiments.
12. Multiple Probes (Optional): – For complex analyses or multicolor FISH (M-FISH), design and label multiple probes to simultaneously target different sequences within the sample.
13. Optimization: – Fine-tune hybridization conditions, such as temperature and probe concentration, to achieve optimal results.
Effective probe design is essential for the success of FISH experiments, ensuring specific and reliable detection of target sequences within cells or tissues. Careful consideration of sequence specificity, length, labeling, and in silico analysis contributes to accurate and meaningful FISH results.
Hybridization Process:
The hybridization process in Fluorescence In Situ Hybridization (FISH) is a critical step where fluorescently labeled DNA or RNA probes bind to their complementary target sequences within the sample. This process allows researchers to visualize and locate specific genetic sequences. Here’s a detailed overview of the hybridization process in FISH:
1. Probe Preparation:
- Start with the preparation of the DNA or RNA probes specific to the target sequences of interest.
- Label the probes with fluorescent molecules (fluorophores) for subsequent detection.
2. Sample Preparation:
- Prepare the sample containing the cells or tissue sections to be analyzed using FISH.
- Fix the sample to preserve cellular structures and prevent degradation.
- If using formalin-fixed and paraffin-embedded (FFPE) samples, deparaffinize and rehydrate them.
- Denature the sample to expose the target DNA or RNA strands.
3. Probe Application:
- Apply the fluorescently labeled probes to the denatured sample.
- Mix the probe solution with a hybridization buffer to create the hybridization mixture.
- Ensure the probe solution covers the entire sample evenly.
4. Hybridization Incubation:
- Incubate the sample with the probe mixture under controlled conditions. Common conditions include:
- Temperature: Typically 37-42°C, depending on the probe and target sequences.
- Duration: The incubation time can vary but usually ranges from several hours to overnight.
5. Probe Binding:
- During incubation, the probes seek out and bind (hybridize) to their complementary sequences within the sample’s DNA or RNA.
- The binding occurs through hydrogen bonding between complementary base pairs (A-T and G-C).
6. Stringency Control:
- Stringency control is essential to ensure specific probe-target binding.
- Stringency is controlled by adjusting temperature, salt concentration, and wash conditions.
- Higher stringency conditions reduce non-specific binding.
7. Post-Hybridization Washes:
- After hybridization, wash the sample to remove unbound probes and minimize background fluorescence.
- Use buffers with specific stringency levels to achieve the desired specificity.
8. Counterstaining (Optional):
- To visualize cellular structures, nuclei, or other components, counterstaining can be performed using dyes like DAPI (4′,6-diamidino-2-phenylindole).
9. Mounting:
- Apply an anti-fade mounting medium to preserve the fluorescence and protect the sample from photobleaching.
10. Microscopy and Imaging: – Examine the sample under a fluorescence microscope equipped with appropriate filters for the fluorophores used in the probes. – Illuminate the sample with specific wavelengths of light to excite the fluorescent labels. – Capture images of the emitted fluorescent signals.
11. Image Analysis: – Analyze the acquired images to determine the location, quantity, and distribution of the target sequences within the sample. – Use specialized software for image processing and quantification if necessary.
Detection and Imaging:
After the hybridization process, the next key steps in Fluorescence In Situ Hybridization (FISH) involve detecting and imaging the fluorescent signals produced by the labeled probes binding to their target sequences within the sample. Here’s an overview of the detection and imaging process in FISH:
1. Fluorescence Microscopy:
- Use a fluorescence microscope equipped with appropriate filters for the specific fluorophores used to label the FISH probes.
2. Illumination:
- Illuminate the sample with specific wavelengths of light that excite the fluorescent labels (fluorophores).
- Each fluorophore has a unique excitation wavelength.
3. Emission of Fluorescent Signals:
- When excited by the appropriate wavelength of light, the fluorophores emit fluorescent signals at their characteristic wavelengths.
4. Filter Selection:
- Use fluorescence filters to selectively capture emitted light from the fluorophores while blocking other wavelengths.
- Filter sets consist of excitation and emission filters that match the fluorophore’s spectral characteristics.
5. Image Acquisition:
- Capture digital images of the sample using a camera attached to the fluorescence microscope.
- Take multiple images at different focal planes if the sample is three-dimensional.
6. Exposure Time and Sensitivity:
- Adjust exposure times and sensitivity settings to optimize image quality and signal-to-noise ratio.
7. Multichannel Imaging (Optional):
- If using multiple fluorophores to label different probes, capture images for each channel (fluorophore) separately.
- This allows you to differentiate and visualize multiple target sequences simultaneously.
8. Image Stacking (Z-Stack):
- If imaging a three-dimensional sample, capture a series of images at different focal planes.
- Combine these images to create a stacked or 3D image, providing a comprehensive view of the sample.
9. Image Processing and Analysis:
- Process the acquired images using specialized software for tasks such as deconvolution, background subtraction, and noise reduction.
- Analyze the images to quantify signal intensity, localization, and distribution of the target sequences within the sample.
10. Visualization and Interpretation: – Visualize the fluorescent signals in the images, which represent the locations of the target DNA or RNA sequences. – Interpret the results based on the distribution and intensity of the signals, correlating them with the research or diagnostic objectives.
11. Data Storage and Documentation: – Store and archive the acquired images and associated data for future reference and reporting. – Document the experimental details, including imaging settings and conditions.
Advantages and Limitations:
Advantages:
- High Specificity: FISH offers exceptional specificity, allowing for the precise detection and localization of target DNA or RNA sequences within cells or tissues.
- Visualization of Genetic Material: It provides visual, direct, and in situ visualization of genetic material, allowing researchers to see where specific genes or sequences are located within the cell.
- Cytogenetic Analysis: FISH is invaluable in cytogenetics for studying chromosomal abnormalities, including deletions, duplications, translocations, and inversions, aiding in the diagnosis of genetic disorders.
- Cancer Research: FISH plays a crucial role in cancer research by identifying genetic alterations associated with tumor development and progression.
- Prenatal and Preimplantation Diagnostics: FISH is used in prenatal and preimplantation genetic diagnostics to detect chromosomal abnormalities in fetuses and embryos, reducing the risk of genetic disorders.
- Microbial Ecology: FISH-ES allows the identification and quantification of microbial species in environmental samples, enhancing our understanding of microbial communities.
- Multicolor FISH (M-FISH): M-FISH enables the simultaneous visualization of multiple target sequences, facilitating complex chromosomal analyses.
- Spectral Karyotyping (SKY): SKY provides a comprehensive view of all human chromosomes in a single experiment, aiding in the diagnosis of chromosomal abnormalities.
- Research Tool: FISH is a versatile research tool used in various fields, including genetics, microbiology, developmental biology, and neuroscience.
- Assisted Reproduction: It assists in selecting embryos with normal chromosomal content during in vitro fertilization (IVF) procedures.
Limitations:
- Limited Resolution: FISH has limited resolution, making it challenging to detect small-scale genetic changes or subtle rearrangements.
- Background Signal: Non-specific binding of probes or autofluorescence can lead to background signals, potentially affecting result accuracy.
- Probe Design: Designing specific and effective probes can be complex, requiring careful consideration of factors such as sequence specificity and melting temperature.
- Hybridization Optimization: Achieving optimal hybridization conditions can be time-consuming and may require experimentation to reduce non-specific binding.
- Sample Complexity: Complex samples with multiple genetic elements or structural variations can complicate FISH analysis.
- Limited Quantification: FISH is primarily a qualitative technique, and quantification may be challenging, especially for low-level target sequences.
- Cell Permeability: In some samples, achieving probe penetration may be difficult due to impermeable cell membranes or tissue structures.
- Cost and Resources: FISH can be resource-intensive, requiring specialized equipment and fluorescently labeled probes, which can be expensive.
- Sample Preparation Variability: Variability in sample preparation can impact FISH results, requiring strict adherence to protocols.
- Expertise Required: FISH analysis requires trained personnel with expertise in probe design, experimental setup, and image analysis.
Variants and Advanced Techniques:
Fluorescence In Situ Hybridization (FISH) has evolved over the years, leading to various variants and advanced techniques that offer enhanced capabilities and applications. Here are some notable variants and advanced techniques of FISH:
- Multicolor FISH (M-FISH):
- M-FISH uses a combination of differently labeled probes to simultaneously visualize multiple target sequences within a single sample.
- Each chromosome or target is assigned a unique color, enabling the identification of complex chromosomal abnormalities.
- Spectral Karyotyping (SKY):
- SKY is an advanced form of FISH that employs a spectrally distinct set of fluorophores to visualize all human chromosomes in a single hybridization experiment.
- It allows for the detection of complex chromosomal rearrangements and structural abnormalities.
- Fluorescence In Situ Hybridization-Fluorescence Activated Cell Sorting (FISH-FACS):
- FISH-FACS combines FISH with flow cytometry to sort and analyze cells based on their DNA content and specific DNA sequences.
- It is valuable in studying cell populations with heterogeneous genomes, such as cancer cells.
- Fluorescence In Situ Hybridization-Immunohistochemistry (FISH-IHC):
- FISH-IHC combines FISH with immunohistochemistry to simultaneously detect genetic and protein markers within the same tissue section.
- This technique provides insights into the correlation between genetic alterations and protein expression in disease states.
- Comparative Genomic Hybridization (CGH) and Array CGH:
- CGH, including array CGH, is a high-resolution technique that compares the DNA copy number variations between normal and diseased cells.
- It is used to identify genomic imbalances associated with diseases, including cancer.
- Quantitative FISH (Q-FISH):
- Q-FISH involves the use of specialized probes and image analysis techniques to quantitatively measure the copy number and telomere length of specific DNA sequences.
- It is applied in aging research, cancer studies, and telomere-related diseases.
- FISH with Locked Nucleic Acid (LNA) Probes:
- LNA probes incorporate modified nucleotides called locked nucleic acids, which enhance probe binding specificity and stability.
- LNAs are particularly useful in challenging FISH applications requiring high stringency conditions.
- RNA FISH (Fluorescence In Situ Hybridization for RNA):
- RNA FISH is used to visualize and localize specific RNA molecules within cells.
- It provides insights into gene expression patterns, RNA localization, and the study of non-coding RNAs.
- Digital FISH (dFISH):
- Digital FISH involves the use of nanoscale materials and advanced imaging techniques to achieve higher sensitivity and resolution in FISH experiments.
- It is used in single-molecule FISH (smFISH) for single-cell RNA analysis.
- Fiber FISH:
- Fiber FISH is used to visualize and measure the length and distribution of extended DNA or chromatin fibers.
- It aids in the study of chromosomal organization and structural changes.
Research and Clinical Applications:
Fluorescence In Situ Hybridization (FISH) has a wide range of research and clinical applications, spanning various fields of biology and medicine. Here are some key research and clinical applications of FISH:
Research Applications:
- Gene Mapping and Localization: FISH is used to map the location of specific genes on chromosomes, helping researchers understand the genetic basis of traits and diseases.
- Cytogenetics: FISH is a cornerstone technique in cytogenetics, allowing the detection of chromosomal abnormalities such as translocations, deletions, duplications, and inversions.
- Cancer Research: FISH is extensively employed in cancer research to identify genetic alterations associated with various cancers. It aids in understanding cancer development, prognosis, and treatment options.
- Genomic Imbalances: Comparative Genomic Hybridization (CGH) and array CGH use FISH to compare DNA copy number variations between normal and diseased cells, revealing genomic imbalances in diseases like cancer and developmental disorders.
- Telomere Analysis: FISH is used to study telomere length and stability, which have implications for aging, cancer, and genetic diseases.
- Microbial Ecology: FISH-ES is used to identify and quantify specific microbial species in environmental samples, enhancing the study of microbial communities and their ecological roles.
- Molecular Cytogenetics: FISH complements traditional cytogenetic techniques by providing higher resolution in the analysis of chromosomes and genetic material.
- Plant Genetics: FISH is applied in plant genetics to study chromosomal organization, genetic diversity, and the identification of specific genes or markers.
- Neuroscience: FISH is used to study gene expression patterns in the brain and neuronal tissues, providing insights into neurodevelopment and neurological disorders.
- Developmental Biology: FISH is applied to study the spatiotemporal expression patterns of genes during development, shedding light on embryogenesis and tissue differentiation.
Clinical Applications:
- Clinical Cytogenetics: FISH is routinely used in clinical laboratories to diagnose genetic disorders, such as Down syndrome (Trisomy 21), Prader-Willi syndrome, and Angelman syndrome.
- Cancer Diagnosis: FISH is employed to detect genetic alterations associated with various cancers, aiding in the diagnosis and prognosis of malignancies.
- Prenatal Testing: FISH is used in prenatal diagnostics to identify chromosomal abnormalities in developing fetuses, providing valuable information for expectant parents and healthcare providers.
- Preimplantation Genetic Diagnosis (PGD): FISH is applied during in vitro fertilization (IVF) to screen embryos for chromosomal abnormalities before implantation, reducing the risk of genetic disorders in offspring.
- Infectious Disease Diagnosis: FISH is used to detect and identify specific bacterial or viral pathogens in clinical samples, contributing to the diagnosis of infectious diseases.
- Assisted Reproduction: FISH helps assess the genetic health of embryos in assisted reproductive technologies, ensuring the selection of embryos with normal chromosomal content.
- Clinical Oncology: FISH is used to guide cancer treatment decisions by identifying genetic markers and targetable alterations in tumor cells.
- Neuropathology: FISH is used to diagnose and classify brain tumors based on specific genetic alterations, aiding in treatment planning.
- Hematology: FISH is employed in the diagnosis and monitoring of hematological disorders, including leukemia and lymphoma.
- Clinical Microbiology: FISH is used for the rapid identification of pathogens in clinical samples, facilitating timely treatment.
Advanced FISH Techniques:
Advanced Fluorescence In Situ Hybridization (FISH) techniques have been developed to address specific research and diagnostic needs, offering improved sensitivity, resolution, and multiplexing capabilities. Here are some advanced FISH techniques:
- Quantitative FISH (Q-FISH):
- Q-FISH incorporates specialized probes and image analysis to quantitatively measure the copy number and telomere length of specific DNA sequences.
- It is used to study telomere biology, aging, and diseases related to telomere length.
- Fluorescence In Situ Hybridization-Fluorescence Activated Cell Sorting (FISH-FACS):
- FISH-FACS combines FISH with flow cytometry, allowing the sorting and analysis of cells based on their DNA content and specific DNA sequences.
- It is valuable for studying heterogeneous cell populations and rare cell types.
- Fluorescence In Situ Hybridization-Immunohistochemistry (FISH-IHC):
- FISH-IHC combines FISH with immunohistochemistry to simultaneously detect genetic and protein markers within the same tissue section.
- It provides insights into the correlation between genetic alterations and protein expression in disease states.
- Digital FISH (dFISH):
- Digital FISH employs nanoscale materials and advanced imaging techniques to achieve higher sensitivity and resolution in FISH experiments.
- It is used in single-molecule FISH (smFISH) for single-cell RNA analysis.
- Super-Resolution FISH (SR-FISH):
- SR-FISH techniques, such as STORM (Stochastic Optical Reconstruction Microscopy) FISH, PALM (Photoactivated Localization Microscopy) FISH, and dSTORM (direct STORM) FISH, break the diffraction limit, achieving nanoscale resolution.
- They are used to visualize molecular details within cellular structures.
- Spatial Transcriptomics (ST-FISH):
- ST-FISH combines FISH with spatial transcriptomics technologies to simultaneously visualize RNA expression patterns and their spatial distribution in tissue sections.
- It aids in understanding tissue organization and cell-to-cell interactions.
- Fiber FISH:
- Fiber FISH is used to visualize and measure the length and distribution of extended DNA or chromatin fibers.
- It helps study chromosomal organization and structural changes.
- RNA FISH (Fluorescence In Situ Hybridization for RNA):
- RNA FISH allows the visualization and localization of specific RNA molecules within cells, providing insights into gene expression patterns and RNA localization.
- Single-Molecule RNA FISH (smFISH):
- smFISH techniques enable the detection and quantification of individual RNA molecules in single cells, offering high sensitivity and single-cell resolution.
- Multiplex FISH (mFISH):
- mFISH techniques utilize multiple probes labeled with distinct fluorophores to simultaneously detect and visualize multiple target sequences within a single sample.
- They are valuable for studying complex genetic interactions and gene expression patterns.
- Whole Chromosome Paint FISH (WCP-FISH):
- WCP-FISH uses chromosome-specific paint probes that cover entire chromosomes.
- It aids in the visualization and identification of whole chromosomes or chromosome regions.
FAQs:
1. What is Fluorescence In Situ Hybridization (FISH)?
FISH is a molecular biology technique that uses fluorescently labeled DNA or RNA probes to bind and visualize specific genetic sequences within cells or tissues.
2. How does FISH work?
FISH involves hybridizing fluorescent probes with complementary target DNA or RNA sequences within a sample. When the probes bind to their targets, they emit fluorescence, which is detected and visualized under a fluorescence microscope.
3. What are the primary applications of FISH?
FISH is used for gene mapping, cytogenetics, cancer research, prenatal and preimplantation diagnostics, microbiology, and various other research and clinical applications.
4. What is the difference between DNA FISH and RNA FISH?
DNA FISH involves detecting specific DNA sequences, while RNA FISH detects RNA molecules, providing insights into gene expression patterns.
5. What are the advantages of FISH?
FISH offers high specificity, allowing precise localization of genetic sequences. It is valuable in cytogenetics, cancer research, and prenatal diagnostics, among other applications.
6. What are the limitations of FISH?
FISH has limited resolution, requires specialized probes, and can be resource-intensive. Background signal and probe design challenges are also limitations.
7. What is the role of fluorescence microscopy in FISH?
Fluorescence microscopy is essential in FISH for visualizing and capturing the fluorescent signals emitted by the labeled probes binding to target sequences.
8. Are there advanced FISH techniques?
Yes, advanced FISH techniques include M-FISH, SKY, smFISH, super-resolution FISH, and others that offer improved sensitivity, resolution, and multiplexing capabilities.
9. How is FISH used in cancer diagnosis and research?
FISH is used to identify genetic alterations associated with cancer, aiding in the diagnosis, prognosis, and targeted treatment of various malignancies.
10. Is FISH used in prenatal testing?
Yes, FISH is used in prenatal testing to detect chromosomal abnormalities in developing fetuses, providing information for expectant parents and healthcare providers.
11. What is the difference between FISH and PCR (Polymerase Chain Reaction)?
FISH visualizes specific DNA or RNA sequences within cells or tissues, while PCR amplifies and detects DNA or RNA sequences in vitro.
12. Is FISH applicable to other organisms besides humans?
Yes, FISH can be applied to study genetic sequences and chromosomal structures in a wide range of organisms, including plants, animals, and microorganisms.
13. Can FISH be used for single-cell analysis?
Yes, techniques like smFISH enable the detection and quantification of individual RNA molecules in single cells, providing single-cell resolution.
14. What are some challenges in FISH experiments?
Challenges include probe design, optimization of hybridization conditions, and minimizing background fluorescence. Sample preparation and image analysis can also be demanding.
15. Is FISH widely used in clinical diagnostics?
Yes, FISH is routinely used in clinical laboratories for diagnosing genetic disorders, detecting cancer-related genetic alterations, and guiding treatment decisions.
Conclusion:
In conclusion, Fluorescence In Situ Hybridization (FISH) is a powerful molecular biology technique that has revolutionized our ability to visualize and study specific DNA or RNA sequences within cells and tissues. Its versatility spans various fields, including genetics, genomics, cytogenetics, cancer research, and clinical diagnostics. By harnessing the principles of hybridization and fluorescence microscopy, FISH enables researchers and clinicians to:
- Map and locate genes and genetic sequences on chromosomes.
- Diagnose and understand chromosomal abnormalities and genetic disorders.
- Investigate cancer-related genetic alterations, aiding in diagnosis and treatment.
- Facilitate prenatal and preimplantation genetic testing.
- Study microbial communities and environmental microbiology.
- Analyze gene expression patterns and RNA localization.
- Advance our understanding of neurobiology, developmental biology, and many other areas of biology.
FISH continues to evolve with the development of advanced variants and techniques, offering higher resolution, sensitivity, and multiplexing capabilities. As technology advances, FISH remains a valuable tool for addressing complex biological questions and providing critical insights into the molecular and genetic basis of health and disease.
As researchers and clinicians harness the potential of FISH and its advanced applications, it is likely to continue playing a pivotal role in scientific discovery, clinical diagnosis, and the advancement of personalized medicine in the years to come.
Possible References Used