Silver Stain: Introduction, Objectives, Principle, Properties, Reagents, Procedure, Applications, Advantages, Limitations.
- Silver staining is a very sensitive method for detecting small amounts of low molecular weight proteins and nucleic acids on polyacrylamide gels.
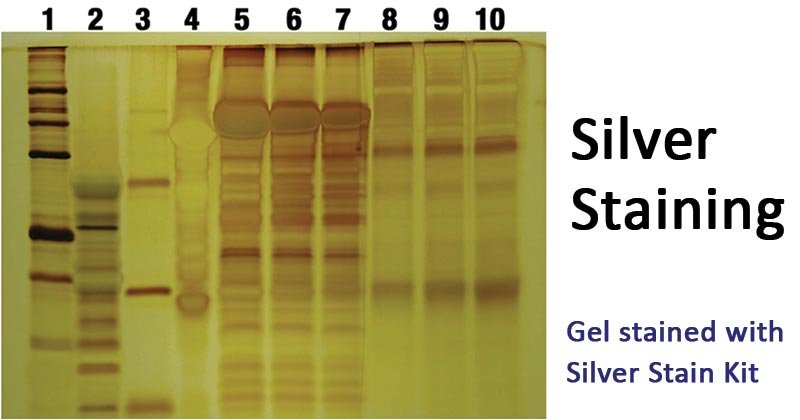
- Silver staining is used to detect proteins after electrophoretic separation on polyacrylamide gels.
- Silver Stain is a fast, ultra-sensitive and versatile silver staining system for the detection of proteins in polyacrylamide gels, producing consistent and reliable results.
- Silver protein staining is an ultrasensitive method for the monochromatic detection of proteins in 1D or 2D polyacrylamide gels with metallic silver ions (Ag).
- Silver staining techniques are widely used to detect nanogram amounts of protein after electrophoresis.
- Silver staining is a rapid and highly sensitive method for detecting proteins and nucleic acids in polyacrylamide gels.
Features:
- Sensitivity: detects proteins at 0.25 ng
- Protocol: gel staining can be performed in 5 minutes to 20 hours, depending on the desired sensitivity
- Gel compatibility: Staining performance is very good with a variety of commercial precast minigels and SDS-PAGE buffer systems.
- Workflow Compatible – Mild chemical formulation ensures compatibility with mass spectrometry, sequencing, and other downstream procedures that rely on protein decolorization and recovery.
Silver staining principle
Silver staining has two main protocols defined by the silver impregnation phase.
- The alkaline protocol
- This method uses a silver nitrate diamine complex in an alkaline space formed by sodium and ammonium hydroxide.
- protein standards are developed in dilute acid solutions of formaldehyde.
- The acid protocol
- This method uses a solution of silver nitrate in water for gel impregnation and the protein standards are developed in a formaldehyde solution in an alkaline environment of ammonia and sodium hydroxide.
- Mainly, the silver staining technique is a simple method that works on the selective reduction of silver at the initiation site close to the protein molecule, to insoluble metallic silver.
- The stages of silver staining are:
- Fixation in which proteins are immobilized and interfering compounds are removed.
- Gel treatment with elements that accelerate the reactivity of proteins to silver and/or silver reduction (sensitization and washing).
- Silver impregnation with simple silver nitrate or ammoniacal silver.
- Silver metal imaging and lightening gel.
- The image tones produced depend on the number of protein bands attached to the silver.
- Silver-stained protein bands appear dark brown or black depending on the intensity of the color of the stained silver.
- Color variations are attributed to diffractions scattered by silver grains of different sizes.
Silver Stain Properties:
- It has a boiling point of 2162 °C (3924 °F)
- It has a melting point of 961.78 °C (1763.2 °F)
- The heat of vaporization: 254 kJ/mol
- It has a density of 10.49 g/cm3
- Molar heat capacity: 25,350 J/(mol K)
Silver staining reagents and solutions
- Sample buffer
- 3% acrylamide solution: prepared by mixing 2.0 mL of 0.8 M Tris-HCl, pH 8.6, 0.75 mL of 38.9% (w/v) acrylamide, and 1.1% bisacrylamide (w/v) in 7.25 ml of water and 8 mg of ammonium persulfate
- 20% acrylamide solution: Prepared by mixing 2.0 mL of 0.8 M Tris-HCl, pH 8.6, 5.0 mL of 38.9% acrylamide, and 1.1% bisacrylamide in 3 mL of water and 8 mg ammonium persulfate)
- Fixation solution prepared by mixing 40% ethanol, 10% acetic acid, 50% water)
- Protein treatment solution is prepared by mixing 20% ethanol, 5% acetic acid, 75% water, 4 mg dithiothreitol
- 0.5% bichromate
- 0.1% silver nitrate
- The complexing solution is prepared with 0.02% paraformaldehyde, 3% sodium carbonate.
- 1% acetic acid
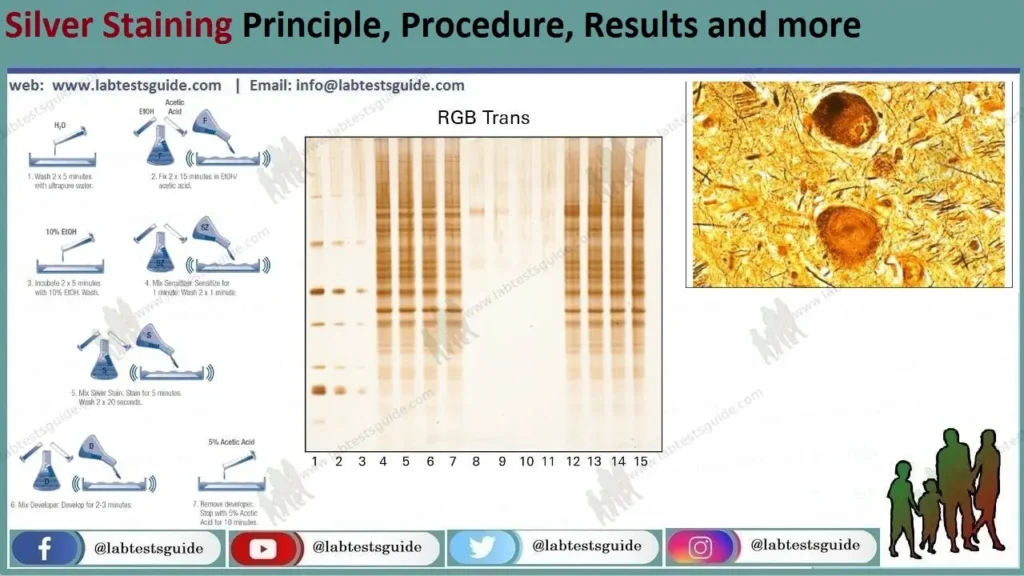
Procedure for silver staining.
- There are variants of protocols of the silver staining technique, but we will discuss two of the most important.
- Initially, a silver staining technique is performed after polyacrylamide gel electrophoresis (SDS-PAGE).
1. Procedure: SDS-PAGE
- Add 20 µg of protein in 10 µL of sample buffer and leave for 60 min at room temperature before separation.
- Fill 8 mL of 3% acrylamide solution and 20% acrylamide solution using a gradient mixer. Pump the solution at a flow rate of 5 mL/min into a glass cuvette.
- Load protein samples onto the gel using a scanning gel, preferably phenol red.
- Run the SDS-PAGE gel at 4 °C and an electrophoresis current of 15 mA.
- Calculate protein concentration using bovine serum albumin.
2. A. Procedure : Silver staining
- Add the fixation solution for 30 min to fix the gel.
- Treat the gel with protein treatment solution for 30 min.
- Rinse the gel with 0.5% dichromate for 5 min.
- Wash the gel with water for 5 minutes.
- Equilibrate the gel with 0.1% silver nitrate for 30 min.
- Wash the gel with water for 1 minute.
- Using the complexing solution, incubate the gel at pH 12.
- To stop complex formation, add 1% acetic acid.
- Fix the gels on glass or polyester slides for observation and/or storage.
2. B. Procedure: Long staining with silver nitrate
- After SDS-PAGE, fix the gels in 30% ethanol and 10% acetic acid for 60 min.
- Renew the fixing bath and leave overnight.
- Sensitize the gel using tetrathionate sensitizing solution for 45 min.
- Rinse the gel with 20% ethanol in two parts (twice), at least 10 min for each wash.
- Rinse the gel four times with water, 10 minutes for each wash.
- Impregnate the gel with 12 mM silver nitrate.
- Arrange the silver nitrate-embedded gels in a box half filled with water, basic developer and a box with the stop solution (40 g Tris and 20 ml acetic acid per litre).
- Rinse with deionized water and extract the gel from the silver solution.
- Soak in a water bath for 10 seconds and transfer to basic developer solution.
- Re-dissolve precipitates by shaking the box containing the developer gel.
- After achieving the degree of staining, transfer the gel to the Tris-stop solution for 30 min.
- Wash the gel with water and display or store it.
Silver Stain Applications
- In general, silver staining is and can be used as a diagnostic tool for bacterial and fungal infections, such as infections caused by Pseudomonas app, Treponema palladium, Helicobacter pylori, Legionella, Leptospira, Bartonella, Pneumocystis, Candida, Histoplasma, Cryptococcus. All of these organisms stain in the silver stain.
- It is used to detect and visualize proteins.
- It can be used to identify the structural differences of proteins.
- It can be used quantitatively to define the number of proteins in a sample.
- It can also be used for genomic analysis by detecting DNA and RNA molecules from samples.
- Also used to detect bacterial lipopolysaccharides on SDS-PAGE
- It can be used to detect fungal lipopolysaccharides such as Histoplasms in liver biopsies.
Advantages of silver staining.
- It is simple to do.
- It’s cheaper
- is trustworthy
- is very sensitive
- Its permanence and simplicity makes it better than the fluorescent probe
- It can also be used to stain DNA and RNA.
Disadvantages of silver staining
- It has a high and erratic background.
- It requires a strong protein-protein bond for variability.