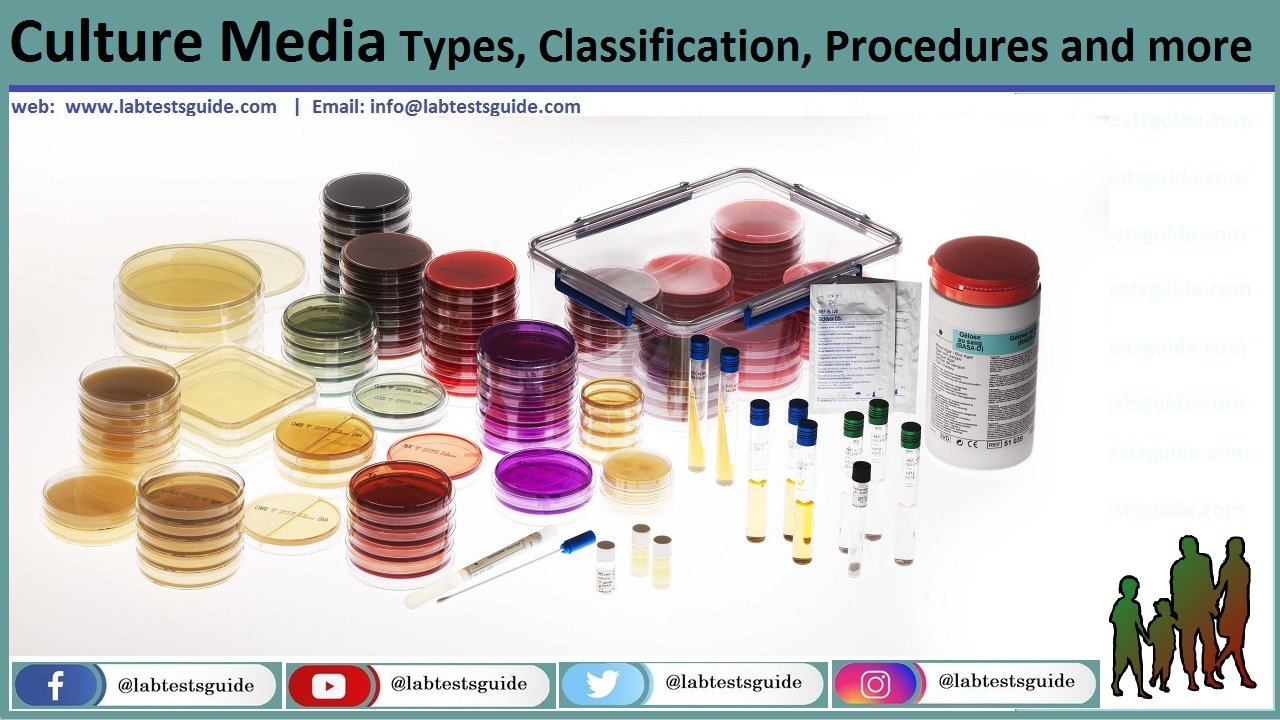
Culture in microbiology refers to the process of growing and maintaining microorganisms, such as bacteria, viruses, fungi, and other microorganisms, in a controlled environment. This controlled environment provides the necessary nutrients, temperature, pH, and other conditions that allow these microorganisms to grow and reproduce.
Introduction
Microorganisms like all other living organisms require basic nutrients for their growth and development. The nutrient preparation on or in which the microorganisms are grown in laboratory is called culture media and the process is called culture. A good culture medium contains sources of carbon, nitrogen, hydrogen, oxygen, phosphorous, inorganic salts 7 other growth promoting substances.
Basic Ingredients:
- Water: source of hydrogen and oxygen.
- Electrolyte: Sodium chloride or other electrolytes.
- Peptone: It is a complex mixture of partially digested proteins. It contains proteoses, amino-acids, polypeptides, phosphates, minerals (K, Mg) and accessory growth factors like nicotinic acid and riboflavin.
- Meat extract
- Yeast extract
- Agar or Agar agar
- Made up of long chain polysaccharide, obtained from sea weed algae.
- It has no nutritive value.
- Usual concentration used in solid media-1-2% (Japanese agar 2% or NewZealand agar 1.2%).
- It melts at 98°C and solidifies at 42°C.
New Zealand agar has more jellifying property than Japanese agar.
Classification
Bacterial culture media can be classified in different ways; Based on chemical composition, physical state and utility purpose.
1. Classification based on composition
Natural culture media
Those media whose exact chemical composition is not known are called natural culture media. Example:-Milk, urine, meat extracts & infusion peptones.
Semi-synthetic culture media
Those media whose chemical composition is partially known are called as semi-synthetic culture media. Example:- Potato Dextrose agar.
Synthetic culture media (Defined culture media)
Those media which are composed of special substances of known composition are called as synthetic media. Example:-Richard’s solution.
2. Classification based on consistency
- Liquid media
- Semi-solid media
- Solid media
Solid media
Any liquid medium can be rendered solid by the addition of certain solidifying agents. Agar agar (simply called agar) is the most commonly used solidifying agent. It is an unbranched polysaccharide obtained from the cell membranes of some species of red algae such as the genera Gelidium. Agar is composed of two long-chain polysaccharides (70% agarose and 30% agarapectin). It melts at 95°C and solidifies at 42oC, doesn’t contribute any nutritive property, it is not hydrolysed by most bacteria and is usually free from growth promoting or growth retarding substances.
Semi-solid media
Reducing the amount of agar to 0.2-0.5% renders a medium semi-solid. Such media are fairly soft and are useful in demonstrating bacterial motility (U-tube and Cragie’s tube). Certain transport media such as Stuart’s and Amies media are semi-solid in consistency. Hugh & Leifson’s oxidation fermentation test medium as well as mannitol motility medium are also semi-solid.
Liquid media
In liquid medium, bacteria grow producing turbidity/ surface pellicle (Vibrio & Bacillus)/ granular deposits (Streptococci). Culturing bacteria in liquid media has some drawbacks. Properties of bacteria are not visible in liquid media and presence of more than one type of bacteria cannot be detected.
Biphasic media
Sometimes, a culture system comprises of both liquid and solid medium in the same bottle. This is known as biphasic medium (Castaneda system for blood culture). The inoculum is added to the liquid medium and when subcultures are to be made, the bottle is simply tilted to allow the liquid to flow over the solid medium. This obviates the need for frequent opening of the culture bottle to subculture.
Media are prepared in following ways:
- Broth tubes: are tubes containing a liquid medium. A typical nutrient containing broth medium such as Trypticase Soy broth, nutrient broth After incubation, growth may be observed as one or a combination of three forms:
- Pellicle: A mass of organisms is floating on top of the broth.
- Turbidity: The organisms appear as a general cloudiness throughout the broth.
- Sediment: A mass of organisms appears as a deposit at the bottom of the tube.
- Slant tubes: are tubes containing a nutrient medium plus a solidifying agent, agar-agar. The medium has been allowed to solidify at an angle in order to get a flat inoculating surface.
- Stab tubes: (deeps) are tubes of hardened agar medium which are inoculated by “stabbing” the inoculum into the agar.
- Agar plates: are sterile petri plates that are aseptically filled with a melted sterile agar medium and allowed to solidify. Plates are much less confining than slants and stabs and are commonly used in the culturing, separating, and counting of microorganisms.
3. Classification based on functional use or application
Simple/Basal media
Basal media are basically simple media that supports most non-fastidious bacteria. Peptone water, nutrient broth and nutrient agar considered basal medium.
- Peptone water-Peptone + NaCl
- Nutrient broth-Peptone + meat extract + NaCl
Complex media– Added ingredients for growth or bringing out certain property.
Enriched Media
Enriched media are used to grow nutritionally exacting (fastidious) bacteria. Addition of extra nutrients in the form of blood, serum, egg yolk etc, to basal medium makes them enriched media. Blood agar, chocolate agar, Loeffler’s serum slope etc are few of the enriched media.
- Enrichment Media
Enrichment media are liquid media that also serves to inhibit commensals in the clinical specimen. Selenite F broth, tetrathionate broth and alkaline peptone water are used to recover pathogens from fecal specimens. Selectively allows certain organism to grow and inhibit others. Eg- Tetrathionate broth (S. Typhi), Alkaline peptone water broth (APW)- Vibrio, Selenite F broth-Shigella
Selective Media (Solid Media)
Selective media are designed to inhibit unwanted commensal or contaminating bacteria and help to recover pathogen from a mixture of bacteria. While selective media are agar based, enrichment media are liquid in consistency. Various approaches to make a medium selective include addition of antibiotics, dyes, chemicals, alteration of pH or a combination of these. Thayer Martin Agar used to recover N. gonorrhoeae contains Vancomycin, Colistin and Nystatin.Mannitol Salt Agar and Salt Milk Agar used to recover S.aureus contain 10% NaCl. Potassium tellurite medium used to recover C.diphtheriae contains 0.04% potassium tellurite.
- Selectively allows certain organism to grow and inhibit others
- Lowestein Jensen media- Mycobacterium tuberculosis
- Potassium tellurite (PTA)- Corynebacterium diphtheriae
- Wilson Blair bismuth sulfite medium- salmonella typhi
- Thiosulphate Citrate Bile salt Sucrose agar- Vibrio
Transport Media
Clinical specimens must be transported to the laboratory immediately after collection to prevent overgrowth of contaminating organisms or commensals. This can be achieved by using transport media. Such media prevent drying (desiccation) of specimen, maintain the viability of all organisms in the specimen without altering their concentration.
| Organism | Transport Media |
| Streptococcus | · Pike’s media |
| Neisseria | · Amies, Sturt’s media |
| Vibrio | · Venkatraman Ramakrishnan (VR) fluid, Autoclaved sea water, Carry Blair medium |
| Enteric pathogen | · Carry Blair medium |
| Shigella, salmonella | · Buffered glycerol saline |
| Bordetella | · Modified stuart’s ( with casamino acid)· Mischulow’s charcoal agar· Dacron or calcium alginate swab used |
Differential media/Indicator media
Differential media or indicator media distinguish one microorganism type from another growing on the same media. This type of media uses the biochemical characteristics of a microorganism growing in the presence of specific nutrients or indicators (such as neutral red, phenol red or methylene blue) added to the medium to visibly indicate the defining characteristics of a microorganism.
- MacConkey agar
- Cystine Lactose Electrolyte Deficient agar (CLED)

- Anaerobic media
Anaerobic bacteria need reduced oxidation –reduction potential and extra nutrients. Such media may be reduced by physical or chemical means. Boiling the medium serves to expel any dissolved oxygen. Addition of 1% glucose, 0.1% thioglycollate, 0.1% ascorbic acid, 0.05% cysteine or red hot iron filings can render a medium reduced.
- Robertson cooked meat that is commonly used to grow Clostridium spps medium .
- Thioglycollate broth contains sodium thioglycollate, glucose, cystine, yeast extract and casein hydrolysate.
- Methylene blue or resazurin is an oxidation-reduction potential indicator that is incorporated
Examples of special Media (E-enrichment, S- selective, D- Differential media)
| Organism | Medium |
| Enteric pathogens- for Salmonella Shigella | · Hektoen enteric agar (S)· Xylose- lysine-dexycholate agar (S)· Deoxycholate citrate agar(S)· Eosin Methylene blue agar (S)· MacConkey (D & S)· Salmonella Shigella agar (S)· Wilson Blair Bismuth Sulfite Media for Salmonella (S)· Selenite F broth (En) , Tetrathionate broth (En) |
| Blood culture- for blood borne pathogens | · Castaneda’s biphasic media (E)- Brain heart infusion agar slope & broth |
| Vibrio cholera (like alkaline growth medium) | · TCBS (Thiosulfate Citrate bile salt sucrose agar (S)· Mansour’s gelatin Taurocholate trypticase agar (S)· Alkaline Bile salt agar (S)· APW- Alkaline peptone water (S) |
| S. aureus | · Mannitol salt agar (s)· Milk salt agar (S)· Ludlam’s medium (S) |
| Streptococcus | · Crystal violet blood agar (S) |
| Nesseria | · Chocolate agar (E),· Thayer- martin media (S)· Modified New York medium (S) |
| Corynebacterium | · Loffler’s serum media (E)· Potassium Tellurite agar (S) |
| Bacillus anthracis | · PLET- Polymxin Lithium EDTA Thallous acetate (S) |
| Bacillus cereus | · MYPA- mannitol egg yolk phenol red polymyxin agar (S) |
| Anaerobes | · Thiglycollate broth Robertson cooked meat broyh |
| Listeria | · PALCAM agar (S) |
| Pseudomonas | · Cetrimide agar (s) king’s media (for pigment) |
| Burkholderia | · Ashdown’s medium (S) |
| Heamophilus | · Blood agar with S. aureus streak (E)· Chocolate agar (E)· Levinthal’s medium (E), Fildes agar (E) |
| Bordetella | · Regan low media (S)· Bordet Gengou Glycerin potato blood agar (E)· Lacey’s DFP media (P) |
| Mycobaterium | · Lowenstein Jensen, Dorset Egg (S) |
| Leptospira | · EMJH (E). fletcher’s (E) Korthoff’s (E) |
| Campylobater | · Skirrow’s Butzer Campy BAP (S) |
| Legionella | · BCYE (Buffered charcoal yeast extract (E) |
| Reiter’s Treponema | · Smith Noguchi media |
Miscellaneous Growth Requirements
| Cholesterol and purines and pyrimidines | · Mycoplasma |
| Cysteine | · Francisella, Brucella, Legionella , Pasteurella |
| X factor (haemin & porphorin) and V factor (NAD) | · Haemophillus influenzae |
| Pyridoxal | · Streptococcus abiotrophia |
Possible References Used