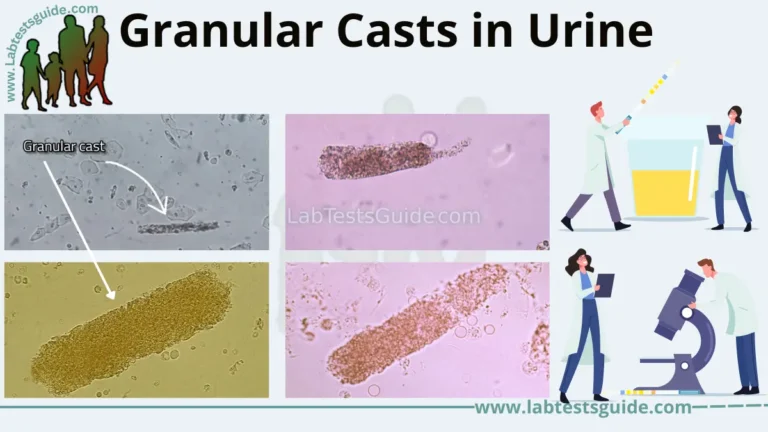
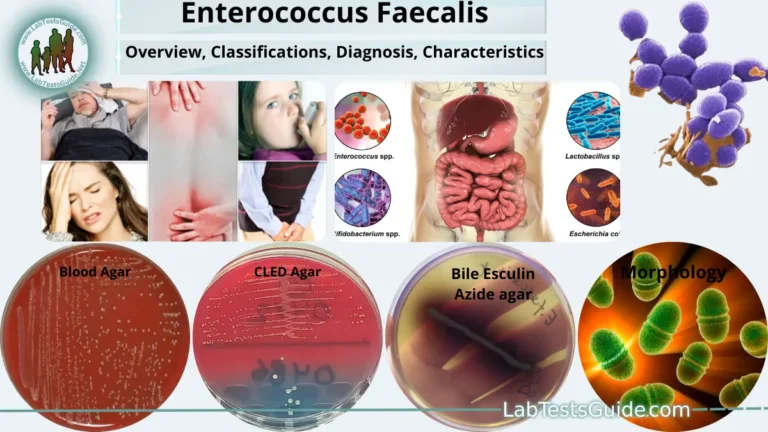
Simmons Citrate Agar is a cornerstone biochemical test in microbiology, used to differentiate enteric bacteria (like Salmonella, Klebsiella, and E. coli) based on their ability to utilize citrate as a sole carbon source. Mastering this test is crucial for accurate pathogen identification in clinical labs.
Key Concepts Explained
- Principle:
- Bacteria metabolize sodium citrate (carbon source) and ammonium phosphate (nitrogen source), producing alkaline byproducts.
- A pH indicator (bromothymol blue) turns blue in alkaline conditions (positive test), while remaining green if citrate is unused (negative test).
- Medium Composition:
- Sodium citrate, ammonium phosphate, magnesium sulfate, agar, and bromothymol blue.
- No carbohydrates – forces bacteria to rely solely on citrate.
- Procedure:
- Streak the slant with a pure bacterial culture.
- Incubate aerobically at 35–37°C for 18–48 hours.
- Avoid caps to ensure oxygenation.
- Interpreting Results:
- Positive: Slant turns Prussian blue (pH >7.6).
- Negative: No color change (green) or growth.
- Note: Growth without color change = negative (alkalinization required).
- Clinical Relevance:
- Klebsiella spp. (positive) vs. E. coli (negative).
- Critical for diagnosing UTIs, GI infections, and sepsis.
Common Pitfalls
- Contamination (non-sterile technique).
- Misreading weak alkalinization (use positive/negative controls).
- Over-incubation causing false positives.
Final Thought
Simmons Citrate Agar is more than a color change—it’s a critical decision-making tool in diagnostics. Revisit this mock test monthly to retain knowledge and refine your lab acumen!