ESR measures the rate (mm per hour) at which erythrocytes sediment in a vertically placed standardized tube (Westergren tube) over 60 minutes. It is a non-specific indicator of alterations in plasma protein composition (primarily fibrinogen and acute-phase reactants) and red blood cell (RBC) aggregation/rouleaux formation. The Westergren Test Method is the reference/gold standard technique for ESR measurement.
| Parameter | Details |
|---|---|
| Test Name | ESR (Westergren Method) |
| Test Definition | Measures erythrocyte sedimentation rate in blood |
| Test Purpose | Non-specific marker of inflammation and plasma protein changes |
| Other Names | Sed Rate, Erythrocyte Sedimentation Rate |
| Test Components | Whole blood, sodium citrate anticoagulant, Westergren tube |
| Pre-Sample Preparation | Collect sample at consistent time; avoid shaking; document medications affecting plasma proteins |
| Fasting Required | Not required |
| Required Sample | Whole blood (citrate 3.8% or 3.2%), 2–3 mL |
| Test Technique | Manual Westergren sedimentation or automated analyzer measurement |
| Normal Values | Adult male: ≤15 mm/h Adult female: ≤20 mm/h Children: 0–10 mm/h Pregnancy: trimester-adjusted (lab validated) |
| Sample Stability | Room temp: up to 4 hours Do not refrigerate or freeze unless validated |

Test Name & Overview
- Test name: Erythrocyte Sedimentation Rate (ESR) — Westergren Test Method
- Overview: Measures the distance (mm) that erythrocytes sediment in a standardized vertical tube over 60 minutes. Reflects plasma protein composition (fibrinogen, acute-phase proteins, immunoglobulins) and erythrocyte properties (size, shape, count) that influence rouleaux formation and sedimentation kinetics. Used as a non-specific marker of systemic inflammation and plasma protein changes.
- Method type: Hematology / gravimetric (physical sedimentation). Not enzymatic, not colorimetric, not molecular; can be manual (Westergren) or automated (instrument-specific methods normalized to Westergren).
Principle of the Westerngren Test:
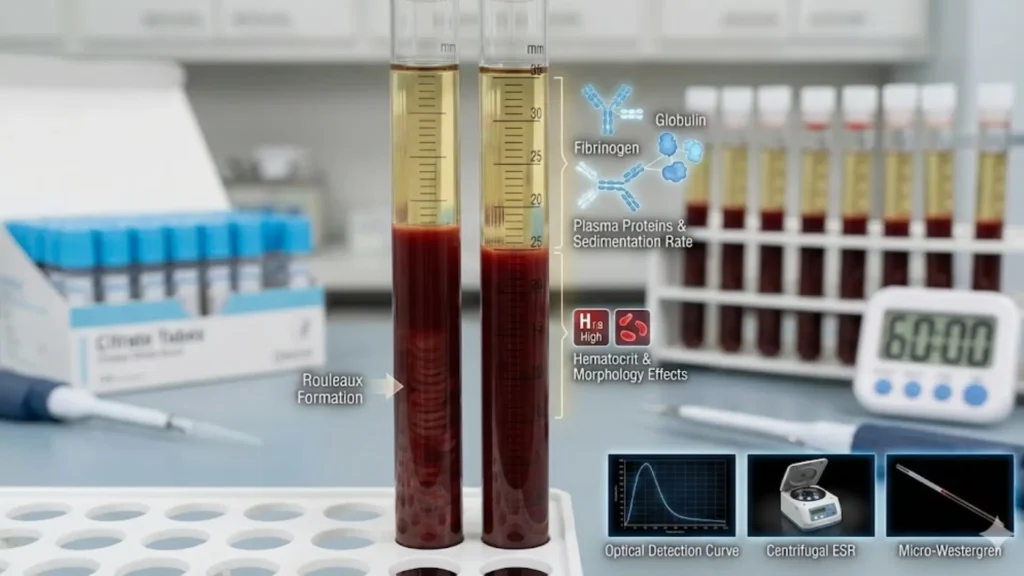
- Westergren (reference) principle: Whole blood anticoagulated with citrate (or diluted appropriately) is placed vertically in a standardized tube. Under gravity, aggregated RBCs (rouleaux) settle; the height of clear plasma (mm) after 60 minutes is recorded as ESR (mm/h).
- Contributors: Plasma fibrinogen, α/β globulins, immunoglobulins ↑ rouleaux → ↑ESR; RBC count/size/shape and hematocrit alter settling.
- Alternate/automated principles (bulleted):
- Automated optical/photometric detection of cell packing/optical density with algorithmic conversion to 60-min equivalent.
- Mechanical or centrifugal accelerated sedimentation with conversion factors to Westergren standard.
- Micro-Westergren or capillary tube methods (validated against full-scale Westergren).
Clinical Significance (Technical):
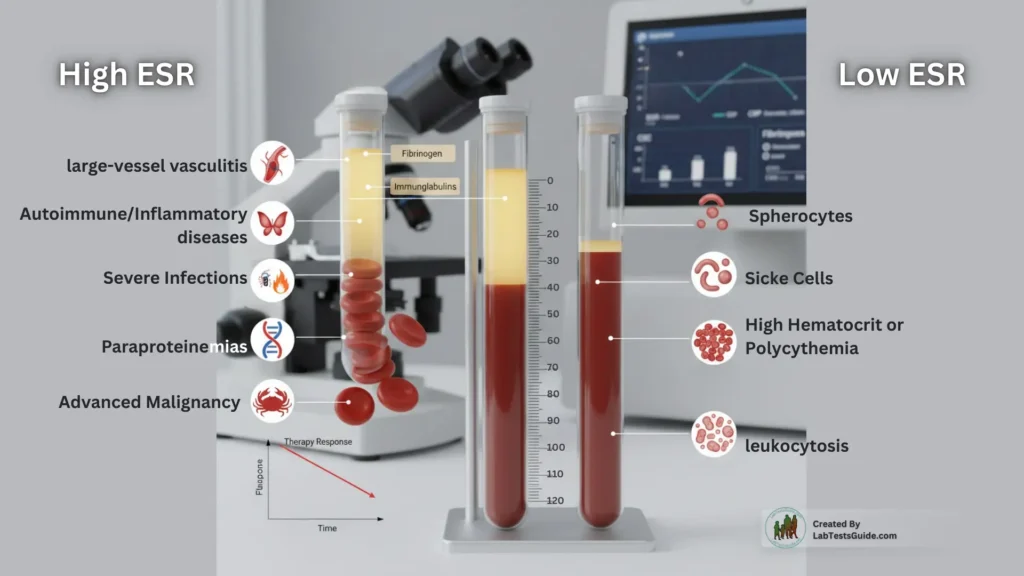
- High ESR — mechanism & relevance: Increased acute-phase proteins (fibrinogen, CRP correlates) enhance rouleaux → faster sedimentation. Seen in large-vessel vasculitis, systemic autoimmune/inflammatory diseases, severe infections, paraproteinemias, advanced malignancy. Use as a trend marker (therapy response) in conjunction with CRP, CBC, fibrinogen. Note ESR rises and falls more slowly than CRP.
- Low ESR — mechanism & relevance: Reduced rouleaux or hindered settling due to abnormal RBC morphology (sickle cells, spherocytes), high hematocrit or polycythemia, marked leukocytosis or hyperviscosity; may mask inflammatory states. Interpret relative to Hct, RBC indices and plasma viscosity.
Specimen Requirements for Westerngren Test
- Specimen type: Fresh whole blood (citrate anticoagulated) or method-validated EDTA with immediate conversion.
- Preferred tube color: Light blue (sodium citrate 3.8% or 3.2%) for direct diluted Westergren. EDTA (lavender) only if SOP validated for EDTA→citrate handling.
- Minimum volume: ~2 mL (depends on tube/tube geometry). For micro methods: 0.5–1 mL as validated.
- Additives: 3.8% (or 3.2%) trisodium citrate (blood:citrate = 4:1 when dilution needed).
- Stability (time & temp): Test as soon as possible; perform within 4 hours at room temperature (20–25 °C) unless local validation supports longer. Avoid refrigeration unless validated.
- Storage & transport: Transport upright, avoid shaking/vibration, maintain ambient lab temperature, protect from extremes. Document collection time.
- Rejection criteria: Clotted sample, gross hemolysis, under/over-filled citrate tube (invalid anticoagulant ratio), inappropriate tube type, samples beyond validated stability, visible fibrin/clot.

| Parameter | Details |
|---|---|
| Test Name | ESR (Westergren Method) |
| Pre-Sample Requirements | • No fasting required • Collect at consistent time for serial monitoring • Document medications affecting plasma proteins (e.g., steroids, IVIG) |
| Specimen | Whole blood (preferred: citrate-anticoagulated) |
| Volume (Ideal) | 2–3 mL |
| Minimum Volume | 1 mL |
| Sample Container | Light blue top tube (3.8% or 3.2% sodium citrate) EDTA tube only if validated by lab |
| Sample Separation & Transport | • Mix gently 3–5× to avoid clotting • Transport upright, avoid vibration • Process within 4 hours at room temperature |
| Sample Storage | Store at room temperature (20–25 °C) if testing within 4 hours. Avoid refrigeration unless validated. |
| Stability Requirements | • Room temperature: up to 4 hours • Do not freeze — RBC morphology compromised • Process immediately for accurate results |
Patient Preparation (Technical):

- Fasting: Not required.
- Timing: For serial monitoring sample at consistent times where possible. Document time.
- Drug restrictions: None required pre-collection; document medications that affect acute phase reactants (e.g., corticosteroids, IVIG, biologics) as they alter ESR kinetics.
- Special conditions: Pregnancy and postpartum physiologically increase ESR — use pregnancy-adjusted ranges.
Reagents & Materials Required:

- Reagents: Sodium citrate 3.8% (or 3.2%) tubes; manufacturer-specified reagents for automated ESR analyzers. SDS available for chemicals.
- Calibrators: Multi-level instrument calibrators traceable to reference, per analyzer.
- Controls: Minimum two levels — Normal (near-reference) and Pathological/High; consider Low/Zero control. Commercial stabilized controls preferred.
- Equipment: Westergren tube/pipette (200 mm), vertical rack/stand, calibrated pipettes, timers, automated ESR analyzer (optional), level surface, thermometer. Centrifuge not required for Westergren.
- Required wavelengths: Not applicable for manual Westergren. For automated optical analyzers, follow manufacturer-specified wavelength(s) (document in method file).
- Reagent storage: Follow manufacturer instructions (lot/expiry logging). Typical: refrigerated 2–8 °C or room temp depending on product.
Westergren Test Procedure (Step-by-Step)
A. Manual (Westergren) — standard (concise):
B. Automated method (concise):
A. Manual (Westergren) — standard:
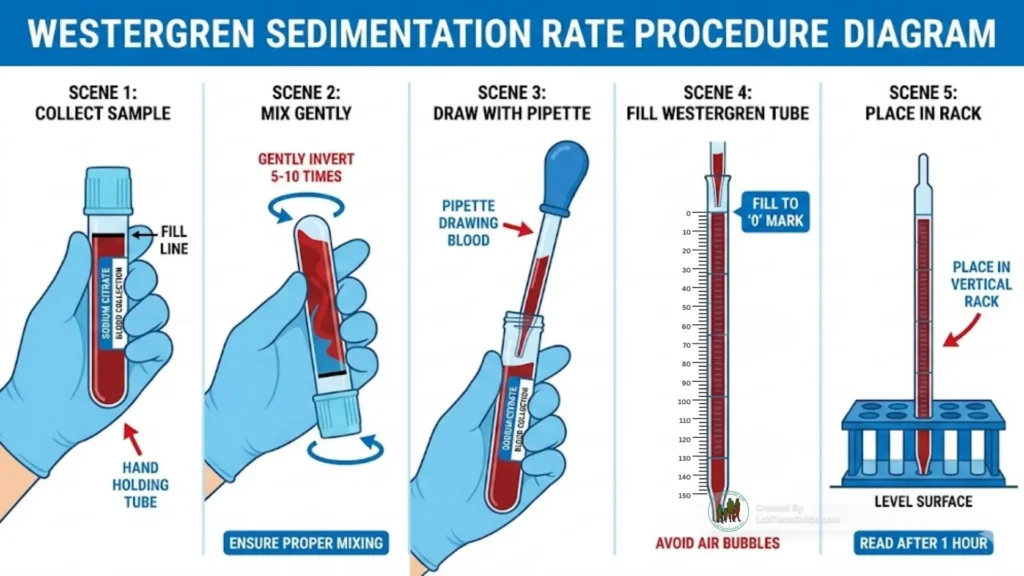
- Label tube/pipette.
- Collect whole blood into citrate tube; invert gently 3–5×.
- Ensure correct blood:citrate ratio (4:1) — fill to mark. If using EDTA, convert per validated SOP.
- Transfer to Westergren tube to 0 mark (no air bubbles). Place vertically in rack on level surface. Start timer immediately.
- Maintain ambient conditions (document temp). Avoid vibration/drafts.
- Read height of plasma column above RBC column at 60 minutes (mm). Record ESR (mm/h). Run QC and only report if QC acceptable.
B. Automated method:

- Analyzer setup: Power on, perform system checks; load reagents, calibrators and controls.
- Reagent loading: Install reagent packs and document lot numbers.
- Calibration frequency: Per manufacturer and validation (initial, reagent lot change, periodic e.g., weekly or as indicated).
- Sample processing: Load patient tubes per analyzer specs (EDTA or citrate as validated). Analyzer measures and reports ESR or 60-min equivalent.
- On-board stability: Follow manufacturer for reagent and sample on-board stability (documented in SOP).
Calculation Formula
- Manual Westergren: ESR (mm/h) = direct reading at 60 minutes (units mm/h).
- Automated / alternate: Use manufacturer’s conversion formula to normalize to 60-min equivalent (device-specific regression or factor).
- Worked example: Plasma clear column measured at 60 min = 42 mm → Report ESR = 42 mm/h.
ESR Westerngren Test Reference Ranges:
Reference ranges must be validated by each laboratory.
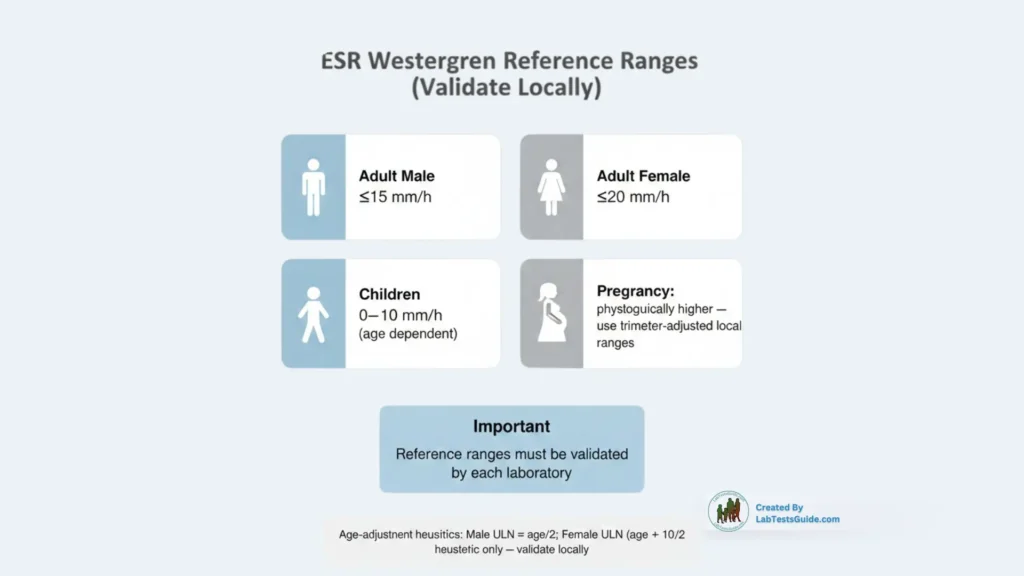
| Population | Typical range (Westergren, mm/h) |
|---|---|
| Adult male | ≤ 15 mm/h (young adults; age-adjustment often applied) |
| Adult female | ≤ 20 mm/h (young adults; age-adjustment often applied) |
| Children | 0–10 mm/h (age dependent) |
| Pregnancy | Physiologically elevated — use trimester-adjusted ranges (local validation) |
Common age-adjustment heuristics: male ULN ≈ age/2; female ULN ≈ (age + 10)/2 — validate locally.
ESR Westergren Test Interpretation (Technical / Professional):
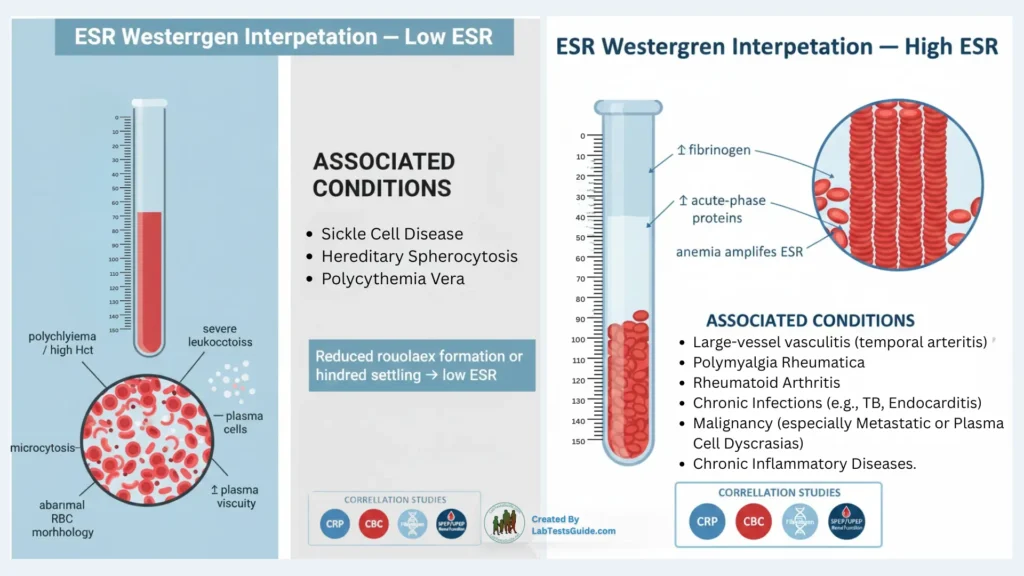
High ESR:
- Mechanisms: ↑ fibrinogen/acute phase proteins → ↑ rouleaux → ↑ sedimentation. Anemia amplifies ESR.
- Associated diseases: Large-vessel vasculitis (temporal arteritis), polymyalgia rheumatica, rheumatoid arthritis, chronic infections (e.g., TB, endocarditis), malignancy (especially metastatic or plasma cell dyscrasias), chronic inflammatory diseases.
- Lab correlations: Compare with CRP, CBC (Hct/Hb), fibrinogen, SPEP/UPEP (for paraproteins), renal function when interpreting systemic disease.
Low ESR:
- Causes: Polycythemia, high Hct, microcytosis, RBC morphological changes (sickle cell, spherocytes), markedly elevated plasma viscosity, severe leukocytosis.
- Mechanism: Reduced rouleaux formation or increased hindered settling.
- Associated conditions: Sickle cell disease, hereditary spherocytosis, polycythemia vera.
Interfering Factors / Sources of Error
- Hemolysis: May alter plasma composition; clotted or hemolyzed samples invalid.
- Lipemia: Can distort automated optical readings; validate manual comparison.
- Icterus / high bilirubin: May interfere with optical automated methods.
- Drugs: Steroids, IVIG, immunomodulators alter acute-phase proteins and ESR kinetics.
- Delayed processing: Leads to inaccurate results — perform within validated stability window.
- Contamination / microclots / fibrin: Cause erratic or low readings.
- Incorrect anticoagulant or ratio: Wrong tube type or wrong blood:citrate ratio invalidates result.
- Temperature variations: Affects plasma viscosity and sedimentation.
- Improper mixing: Inadequate inversion → clotting or uneven anticoagulation.
- Instrument drift / dirty optics: For automated systems, causes bias / erroneous readings.
Quality Control Requirements
- QC levels: Minimum two levels — Normal and High (pathological). Add Low if available.
- Frequency: Daily controls before reporting; increase frequency with high workload or per shift if required by accreditation. Run QC after reagent lot changes, maintenance, or calibration.
- Acceptable ranges: Use control material manufacturer target ± allowable limits established during validation; compute mean and SD.
- Westgard rules: Implement appropriate multi-rule strategy (e.g., 13s as warning, 12s/22s/R4s for rejection). Document chosen rules and actions.
- Levey-Jennings: Plot daily QC; monitor shift/trend and sudden shifts.
- Corrective actions: On QC failure — stop reporting, repeat controls, inspect instrument/reagents, recalibrate if indicated, repeat patient samples only after QC acceptable; document actions.
Calibration Requirements
- Calibration frequency: At installation, after reagent lot change, after major maintenance, and per manufacturer schedule (e.g., initial and periodic). Validate frequency during method validation.
- Multi-level calibrators: Use at least two or three levels spanning clinical range; traceable to reference where possible.
- Linearity limits: Establish during validation (e.g., 0–120 mm/h or manufacturer-specified). Document upper measurement limit and instructions to report “> upper limit” when exceeded.
- Recalibration triggers: Reagent lot change, persistent QC bias/trend, calibration failure, after instrument repair, or significant discrepancy vs reference method.
Instrument Maintenance Notes
- Daily: Run startup self-test; inspect sample probe and wash; run controls; clean spills.
- Weekly: Inspect tubing and reagent packs; empty waste; check leveling of racks; clean exterior surfaces.
- Monthly: Deep clean optical windows/sensors per manufacturer; replace filters if applicable; check calibration status.
- As needed: Replace worn probe tips, perform preventive maintenance per service schedule, update firmware only after validating performance. Document all maintenance.
Reporting Format (Professional example)
- Result: ESR = 42 mm/h
- Unit: mm/h
- Reference range: Adult male ≤ 15 mm/h; Adult female ≤ 20 mm/h (Method: Westergren — lab-validated ranges apply)
- Flag: H (High)
- Method used: Westergren (citrate 4:1) or Automated ESR analyzer — [make/model]
- Notes (technical): Sample in 3.8% sodium citrate; processed within X hours; QC within limits; consider correlating with CRP, Hb, and SPEP if indicated.
Critical (Panic) Values
- Suggested urgent thresholds: ESR > 100 mm/h — notify ordering clinician per local policy. Some labs use ESR >120 mm/h for urgent alert.
- Always: “Follow institutional policy for notification.” Document notification.
Troubleshooting Guide (issue → cause → steps)
- Low absorbance / no signal (automated): Cause — aspiration failure, air in probe. Steps — check sample aspiration, clean probe, re-aspirate, run self-test.
- High blank / background (automated): Cause — dirty optics or contaminated reagents. Steps — clean optical path, replace reagents, run blank and recalibrate.
- Drift / trending bias: Cause — temperature instability, aging reagents, instrument drift. Steps — run controls, verify ambient temp, change reagent lot, recalibrate, contact service if persistent.
- Reagent deterioration: Cause — improper storage/expired. Steps — check storage logs, replace reagents, re-run controls.
- Sample clot observed: Cause — inadequate mixing or wrong tube. Steps — reject sample, request redraw with correct tube and proper mixing.
- Calibration failure: Cause — wrong calibrator, instrument fault. Steps — verify calibrator identity, repeat calibration, service call if failure persists.
- QC out of range: Steps — stop reporting, repeat QC, inspect recent testing, check reagents/maintenance, recalibrate if indicated, release patient results only after acceptable QC and documentation of corrective actions.
Safety Precautions
- PPE: Gloves, lab coat, eye protection when handling blood. Follow bloodborne pathogen protocols.
- Biohazard disposal: Used tubes, tips, and contaminated consumables to biohazard/sharps per institutional regulations.
- Reagent hazards: Follow SDS for citrate and cleaning chemicals.
- Analyzer safety: Follow electrical and reagent handling instructions in instrument manual.
- Contamination prevention: Use single-use consumables where appropriate, perform routine probe washes, avoid cross-contamination.
Test Limitations
- Analytical: ESR is non-specific; automated methods require validation vs Westergren; differing methods not directly interchangeable without conversion. Upper linearity limits vary by method.
- Biological: Influenced by age, sex, pregnancy, hematocrit, RBC morphology, paraproteins, and viscosity.
- Interfering metabolites / conditions: Paraproteinemia, extreme lipemia or icterus may affect some automated measurements unpredictably.
- Cross-reactivity / non-specificity: Not applicable in immunoassay sense — changes reflect multiple possible etiologies.
- Method-specific limits: Always report method used and lab-validated ranges.
Notes for Lab Students (MUST remember)
- Westergren = reference method; read at 60 minutes and report in mm/h.
- Use correct anticoagulant and ratio (citrate 4:1) or follow validated EDTA workflow.
- Preanalytics (mixing, timing, temp, upright transport) critically affect result.
- ESR is non-specific — always correlate with CRP, CBC and clinical context.
- Run QC before reporting and document all actions.
FAQs
What is the reference method for ESR measurement?
The Westergren method is the internationally accepted reference method, endorsed by ICSH. Automated ESR instruments must correlate their results to this standard.
Can EDTA samples be used for Westergren ESR?
Yes, only if the laboratory has validated an EDTA → citrate conversion protocol. Standard Westergren requires citrate (3.8% or 3.2%) with a 4:1 blood-to-anticoagulant ratio.
What is the acceptable time window to perform ESR after collection?
Ideally within 2–4 hours at room temperature (20–25 °C). Beyond this window, rouleaux formation and RBC morphology may change, affecting sedimentation.
Why does anemia increase ESR values?
Lower RBC mass reduces settling resistance, allowing rouleaux to sediment faster. ESR can therefore appear falsely elevated in iron deficiency anemia, hemolytic anemia, and anemia of chronic disease.
Does CRP correlate with ESR?
CRP and ESR often rise together in inflammation, but not always.
CRP responds rapidly to acute inflammation.
ESR responds more slowly and is influenced by hematocrit and plasma proteins.
ESR may remain high even when CRP normalizes.What is the maximum reportable ESR value?
Depends on method:
Manual Westergren: Up to 200 mm/h (tube length 200 mm).
Automated analyzers: Use manufacturer-stated linearity (e.g., 120–140 mm/h).
Readings above linearity are typically reported as “> upper limit.”How do temperature variations affect ESR?
Temperature influences plasma viscosity.
High temperature → faster sedimentation (false ↑ ESR).
Low temperature → slower sedimentation (false ↓ ESR).
Perform at controlled room temperature for best accuracy.Why do paraproteins cause extreme ESR elevations?
Monoclonal immunoglobulins (IgG, IgA) increase plasma viscosity and promote marked rouleaux formation, causing ESR to exceed 100 mm/h even without acute inflammation — common in multiple myeloma and Waldenström macroglobulinemia.
Why does sickle cell disease produce extremely low ESR values?
Sickle-shaped RBCs cannot form rouleaux effectively. Their abnormal morphology and increased rigidity prevent normal sedimentation, producing very low ESR, even during inflammation.
Can ESR be used to monitor therapy response?
Yes, but cautiously:
ESR is best for long-term inflammation monitoring (e.g., temporal arteritis, polymyalgia rheumatica).
Poor for acute changes due to slow response time.
Always correlate trends with CRP, CBC (Hct), fibrinogen and clinical context.What is the standard read time for the Westergren ESR?
60 minutes.
What anticoagulant and ratio is standard for diluted Westergren?
Sodium citrate 3.8% (or 3.2%); blood:citrate = 4:1.
Name two plasma proteins that increase ESR.
Fibrinogen and immunoglobulins (α/β globulins contribute).
How does significant anemia affect ESR and why?
It increases ESR — lower RBC mass reduces hindrance to settling and promotes relatively faster rouleaux sedimentation.
Daily QC fails on the high control (13s). What is your immediate action?
Do not report patient results; repeat QC, check reagents/instrument, correct cause, and only release patient results when QC is acceptable. Document corrective actions.