Acid-base disturbances are pathologic changes in the partial pressure of carbon dioxide (Pco2) or serum bicarbonate (HCO3-) that typically produce abnormal arterial pH values.
- Acidemia is a serum pH < 7.35. Alkalemia is a serum pH > 7.45.
- Acidosis refers to physiological processes that cause acid accumulation or alkali loss.
- Alkalosis refers to physiological processes that cause alkali accumulation or acid loss.
The actual changes in pH depend on the degree of physiological compensation and the presence of multiple processes.
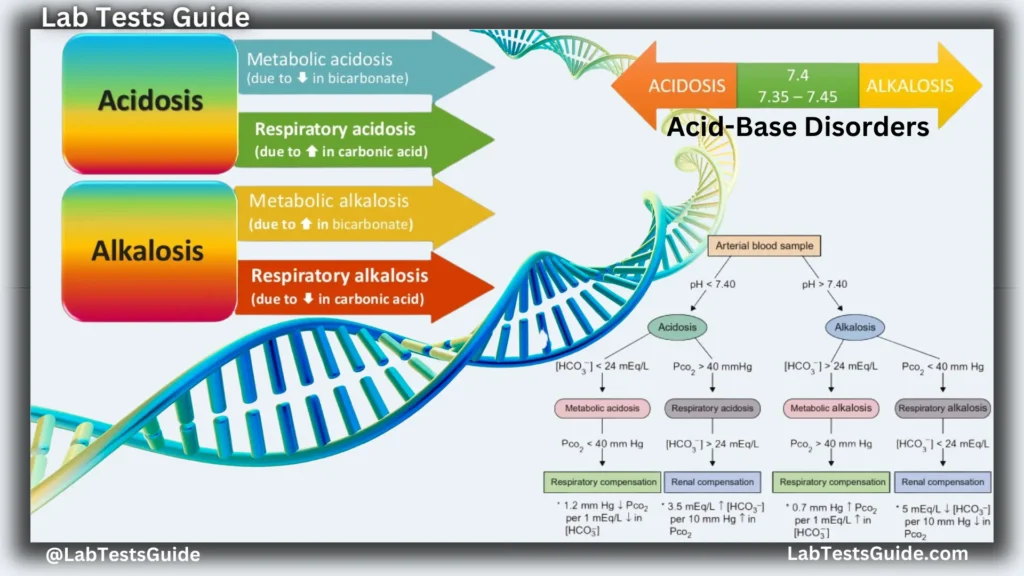
Our bodies are amazing at maintaining a stable internal environment, a state called homeostasis. One crucial aspect of this stability is keeping the blood’s pH level within a tight range. Imagine pH as a measure of acidity or alkalinity, with 7 being neutral. The body carefully regulates pH to ensure most functions run smoothly.
So, how does it do this? The key players are bicarbonate ions (HCO3-) and carbon dioxide (CO2). The Henderson-Hasselbalch equation describes this relationship, showing how pH depends on the ratio of bicarbonate concentration to CO2 tension.
Types:
When this balance is disrupted, we can develop acid-base disorders. There are four main types:
- Metabolic acidosis: This occurs when there’s too much acid in the body or a loss of bicarbonate.
- Respiratory acidosis: This results from the body not being able to eliminate CO2 effectively, causing it to build up.
- Metabolic alkalosis: This happens when there’s an excess of bicarbonate or a loss of acid.
- Respiratory alkalosis: This occurs when the body gets rid of CO2 too efficiently, often due to rapid breathing.
Doctors can diagnose these disorders by measuring blood gas levels and electrolytes. Interestingly, venous blood (from a vein) is usually sufficient unless they also need to assess oxygen levels.
Treatment for acid-base disorders focuses on addressing the underlying cause. However, in severe cases (pH below 7.2 or above 7.6), specific measures might be needed. For instance, severe metabolic acidosis may require sodium bicarbonate. Close monitoring is crucial to prevent overcorrection and alkalosis.
An important note: changes in pH can affect potassium levels in the blood. Therefore, doctors keep a close eye on potassium throughout treatment for acid-base imbalances.
Conclusion:
In conclusion, maintaining a healthy blood pH is essential for our well-being. Understanding acid-base disorders and their causes can help us appreciate the complex systems that keep our bodies in balance. If you have any concerns about your acid-base balance, consult a healthcare professional.