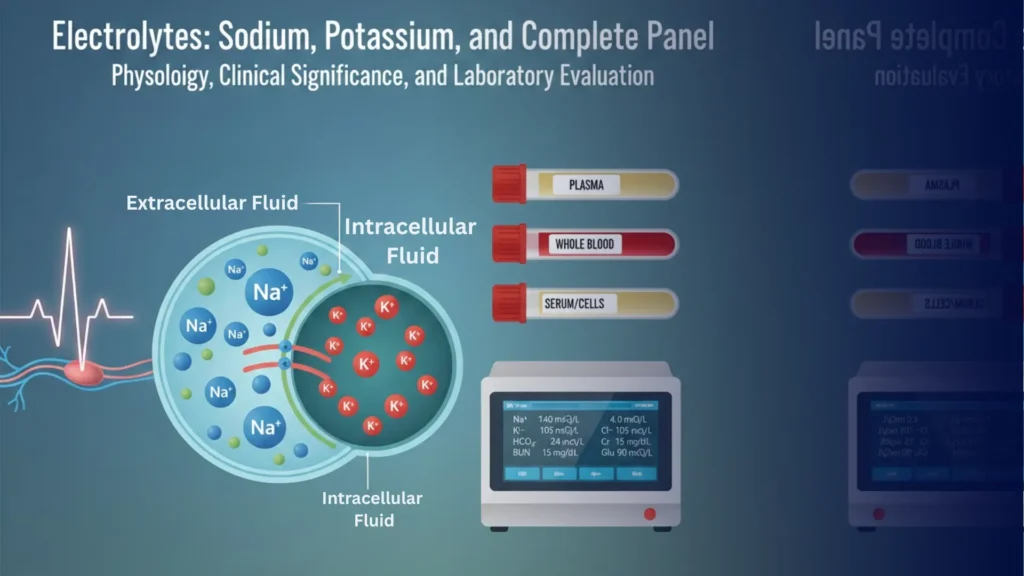
Electrolytes: Sodium, Potassium, and Complete Panel – Physiology, Clinical Significance, and Laboratory Evaluation
Electrolytes are charged low-molecular-weight molecules present in plasma and cytosol. They are essential for maintaining osmotic pressure, acid-base balance, neuromuscular function, and cellular metabolism. In adults, approximately 60% of body weight is water, containing these electrolytes.
Understand Your Test Results:
Understand your Electrolytes Results
AI-powered Lab Test Results Meaning tool 🤖
Major electrolytes include:
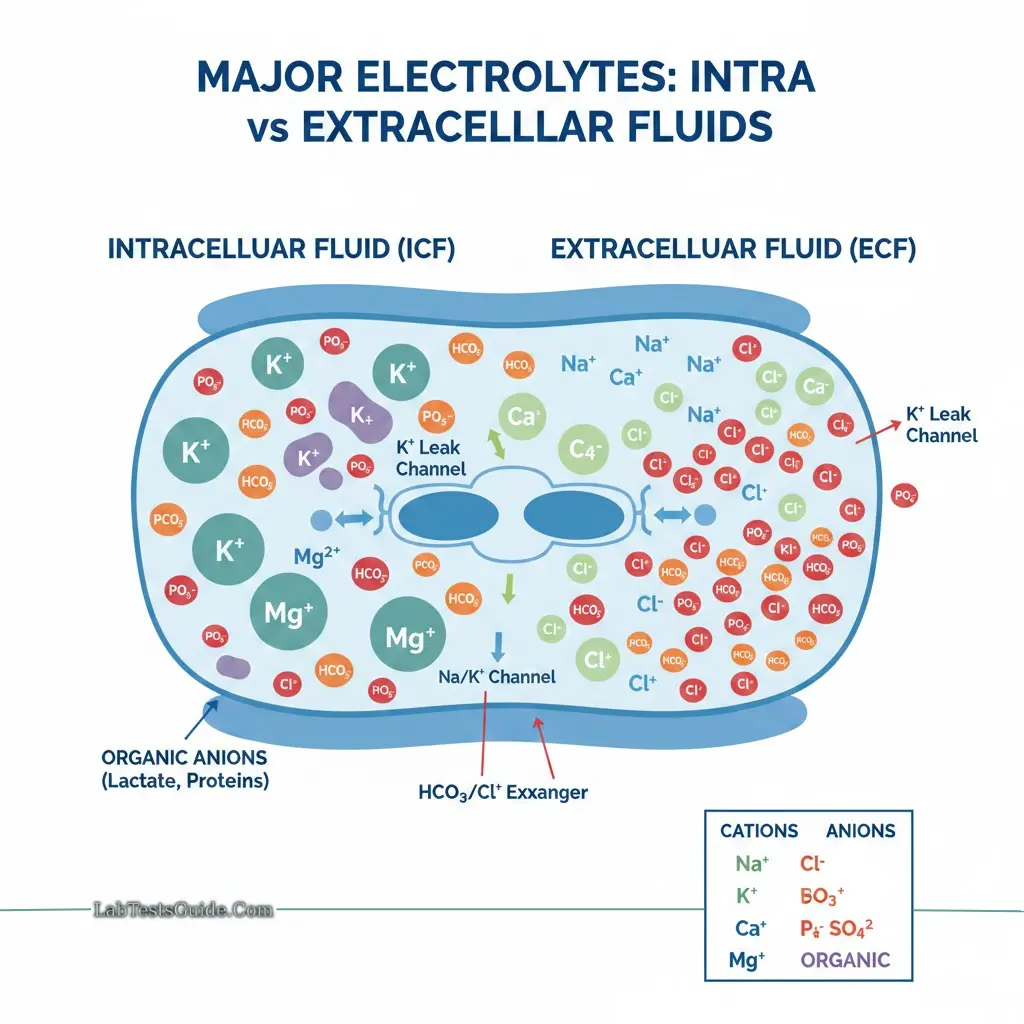
- Cations: Sodium (Na⁺), Potassium (K⁺), Calcium (Ca²⁺), Magnesium (Mg²⁺)
- Anions: Chloride (Cl⁻), Bicarbonate (HCO₃⁻), Phosphate (PO₄³⁻), Sulfate (SO₄²⁻)
- Organic anions: Lactate, trace elements
Electrolytes are divided into:
- Cations: positively charged, move toward the cathode
- Anions: negatively charged, move toward the anode
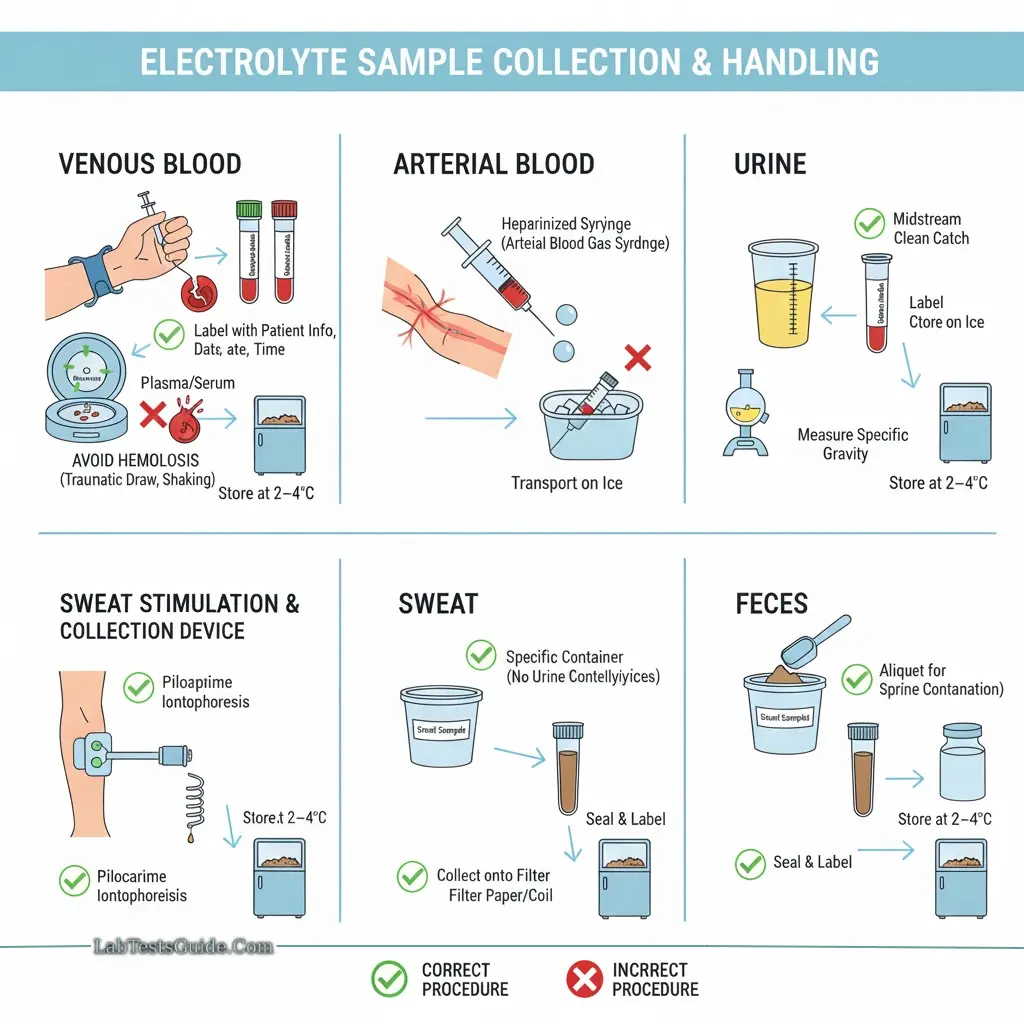
Sample Collection and Handling
Blood: Venous blood is preferred; arterial blood may be used for certain parameters.
Plasma or Serum: Serum is commonly used; plasma is acceptable but may show slightly lower K⁺ values. Separate serum/plasma as soon as possible.
Urine: 24-hour urine collection without preservatives; fractional collection may be used.
Other Samples: Sweat, feces, gastrointestinal fluids.
Precautions:
- Avoid hemolysis, which can falsely elevate potassium.
- Avoid prolonged tourniquet use or repeated fist clenching.
- Lipemic serum must be ultracentrifuged before sodium measurement.
- EDTA tubes should not be used for potassium (contain K⁺).
Potassium (K⁺)
Physiology
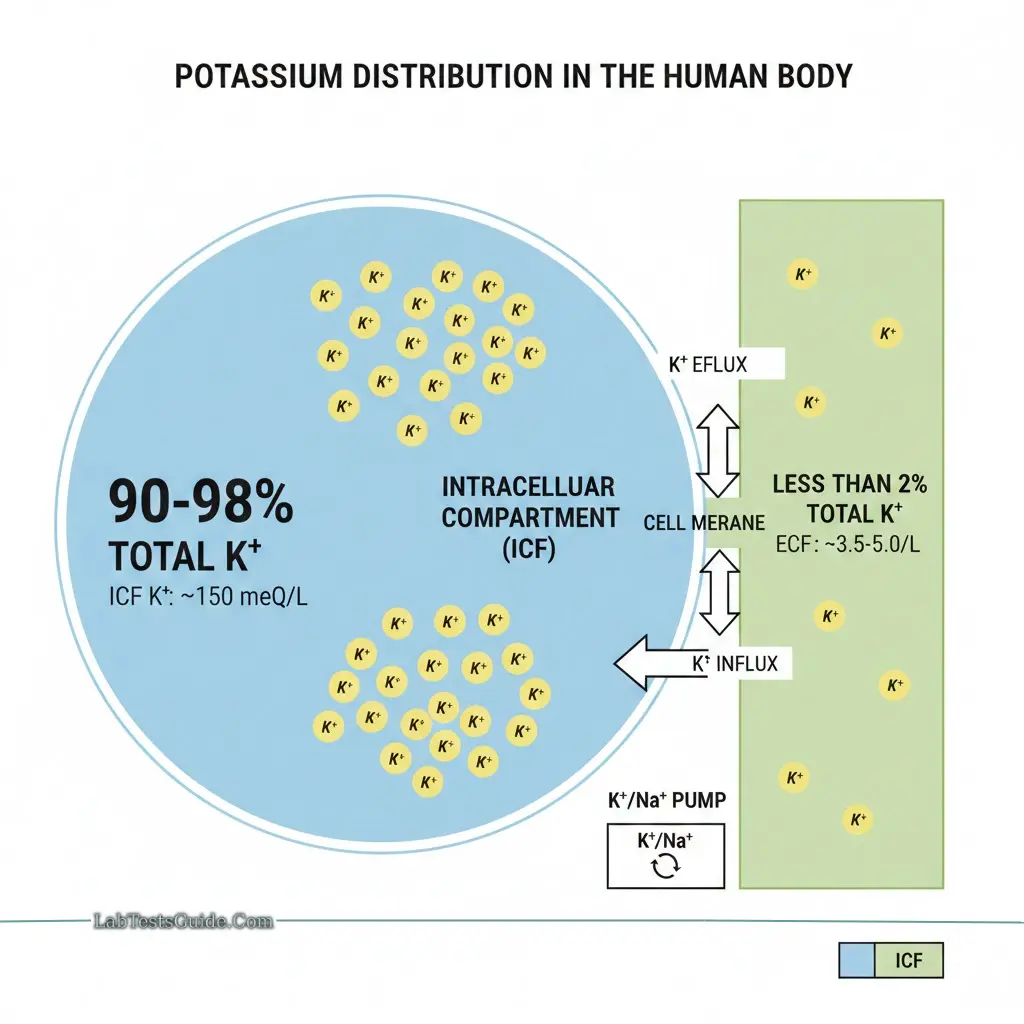
Potassium is the primary intracellular cation, with approximately 90% inside cells and <2% in extracellular fluid. This gradient is critical for membrane potential and neuromuscular conduction.
Daily intake: 40–150 meq/day (average 1.5 meq/kg body weight)
Concentrations:
- Intracellular: 150 meq/L
- Blood: ~4 meq/L
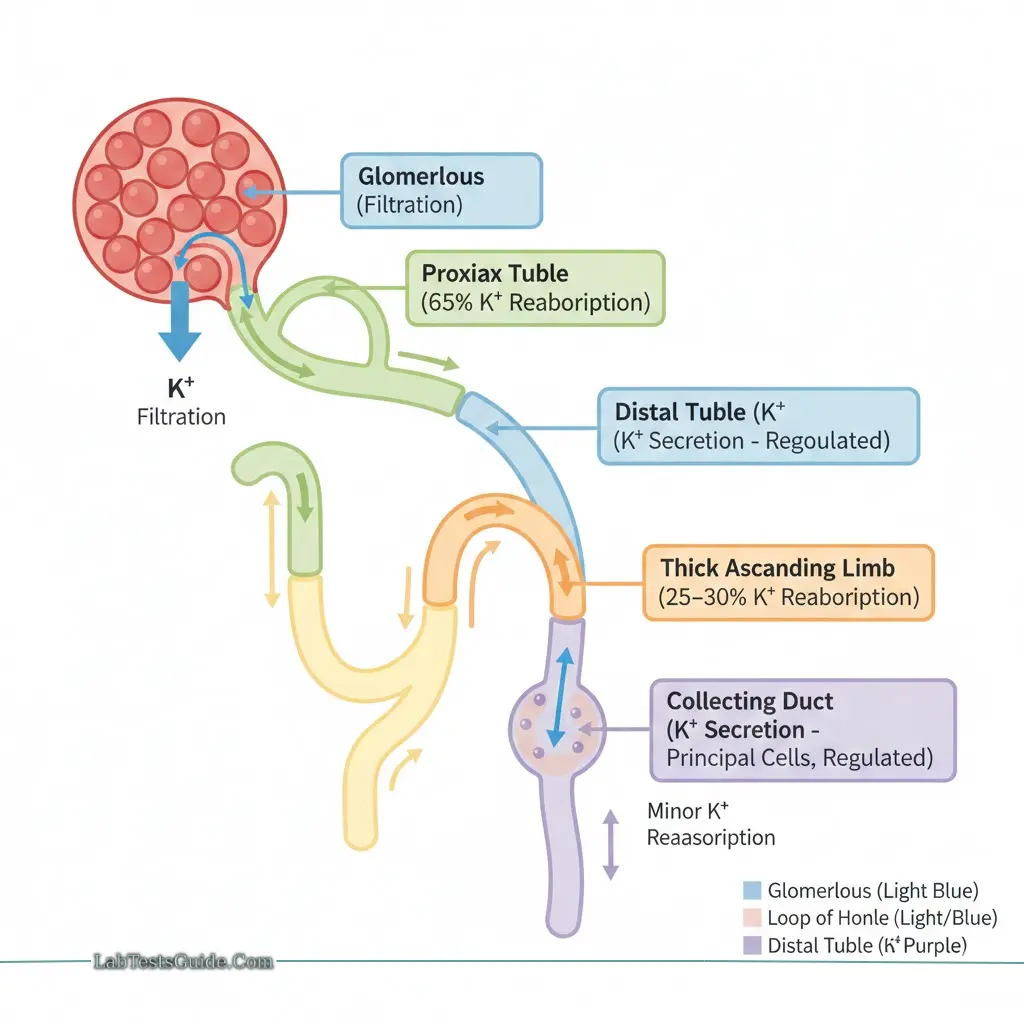
Renal Handling
- 80–90% excreted by kidneys (glomerular filtration, proximal and distal reabsorption)
- 10–20% excreted in sweat and stool
- Aldosterone stimulates renal K⁺ excretion
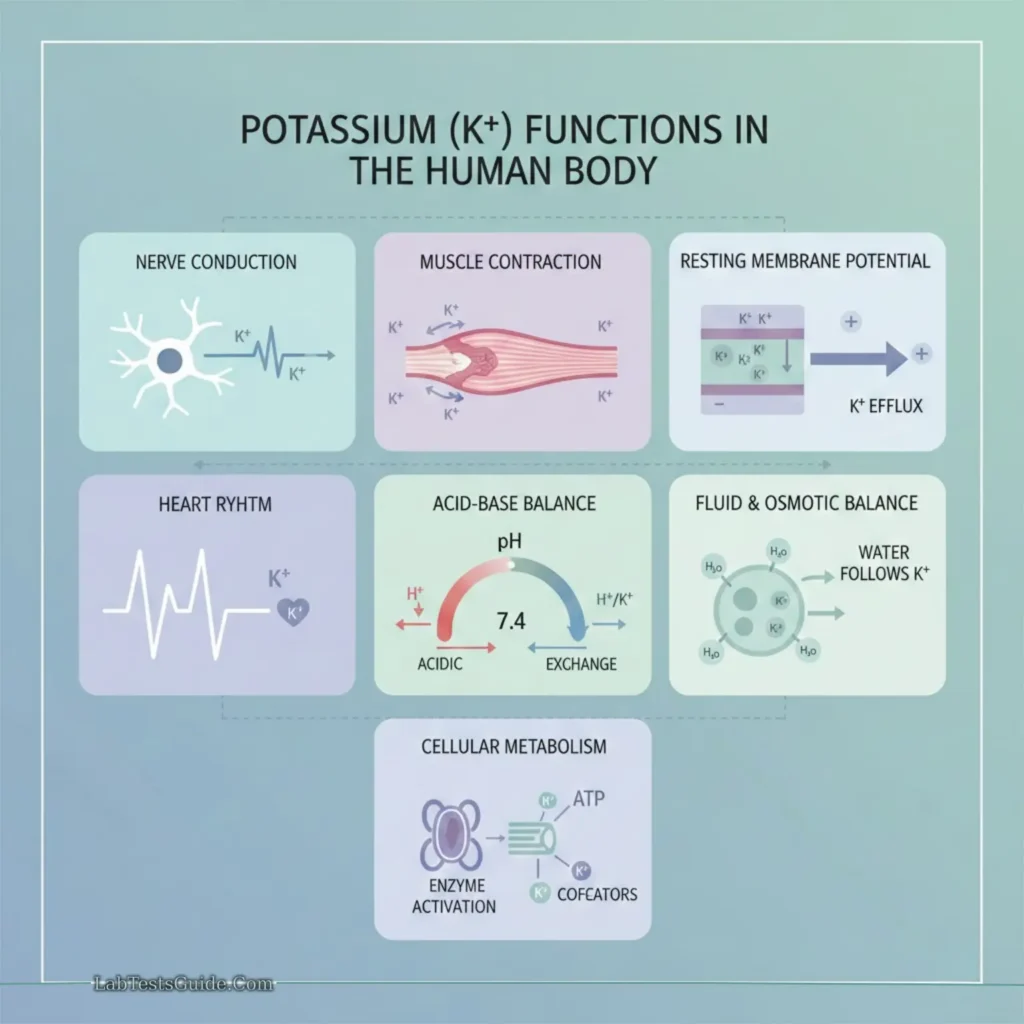
Functions
- Nerve conduction
- Skeletal and cardiac muscle contraction
- Acid-base balance
- Enzyme reactions in carbohydrate and protein metabolism
Pathophysiology
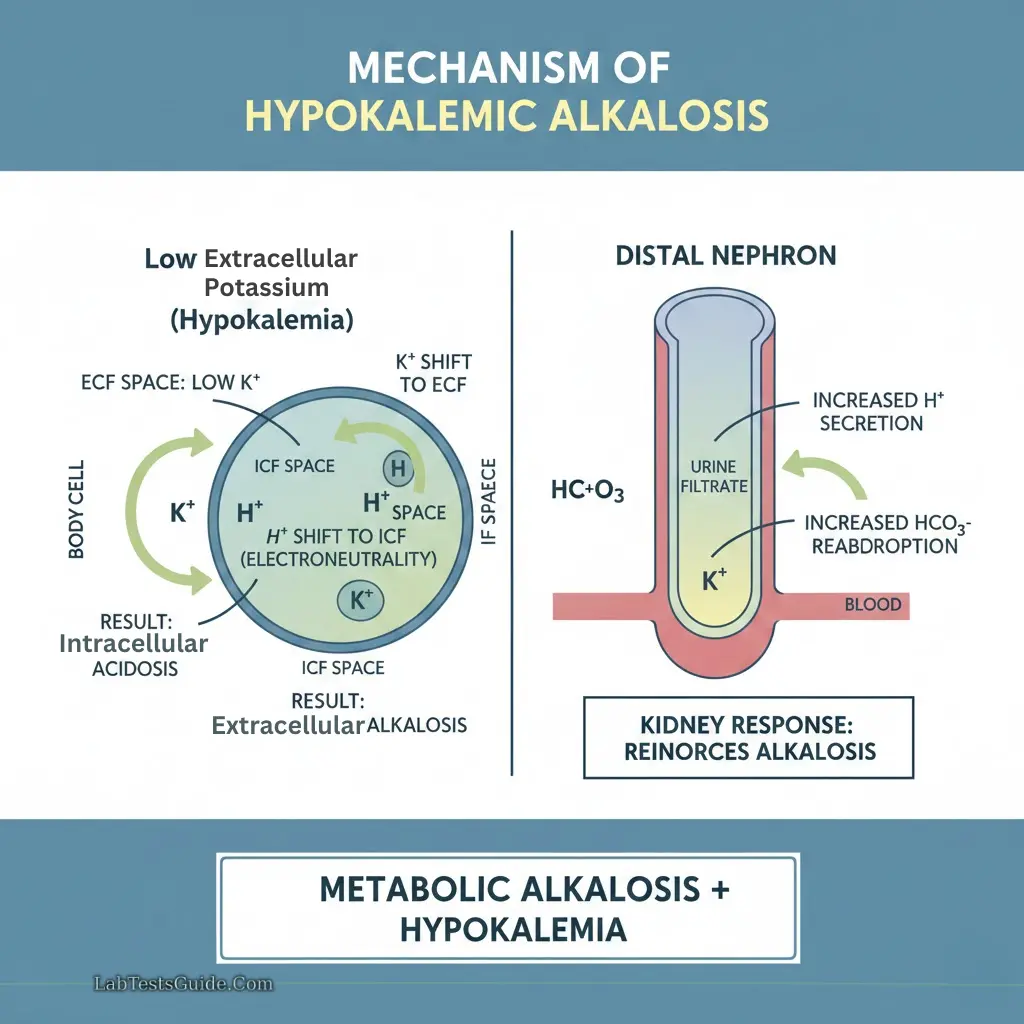
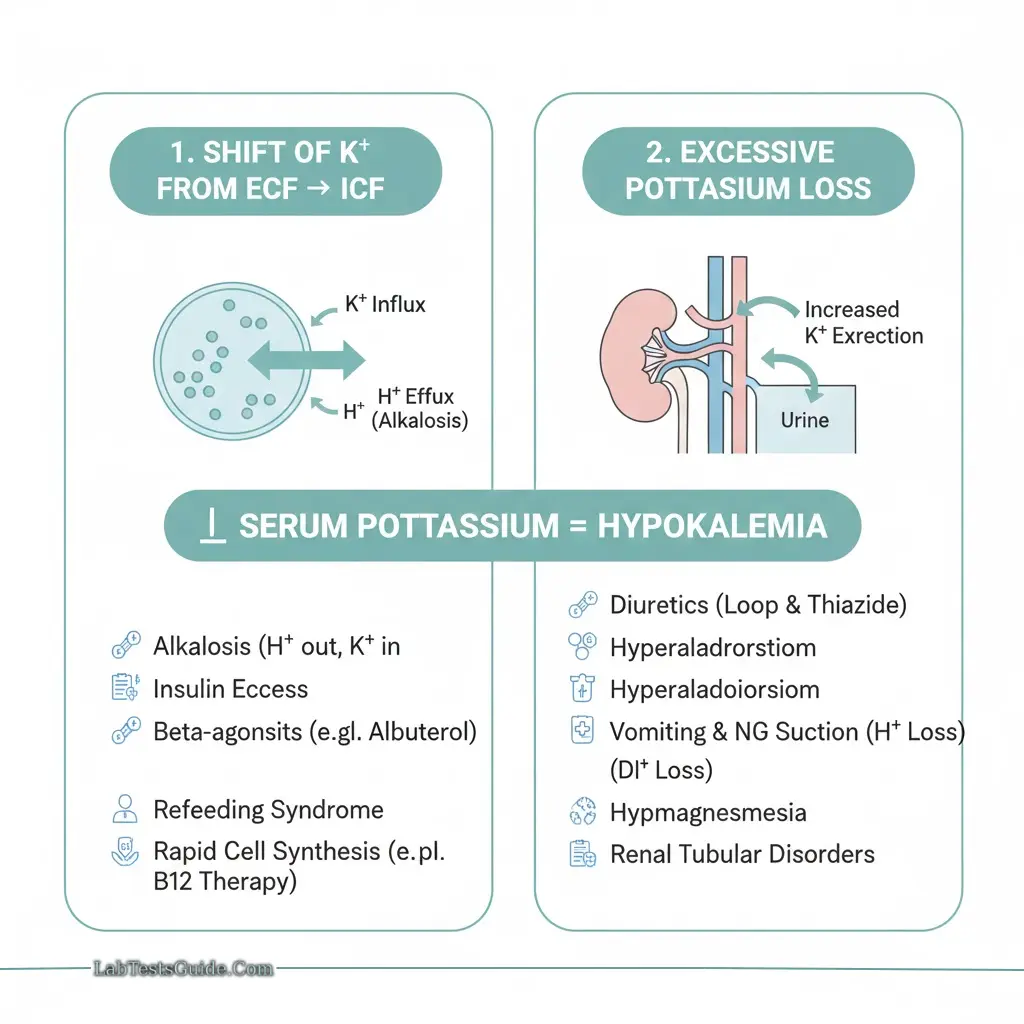
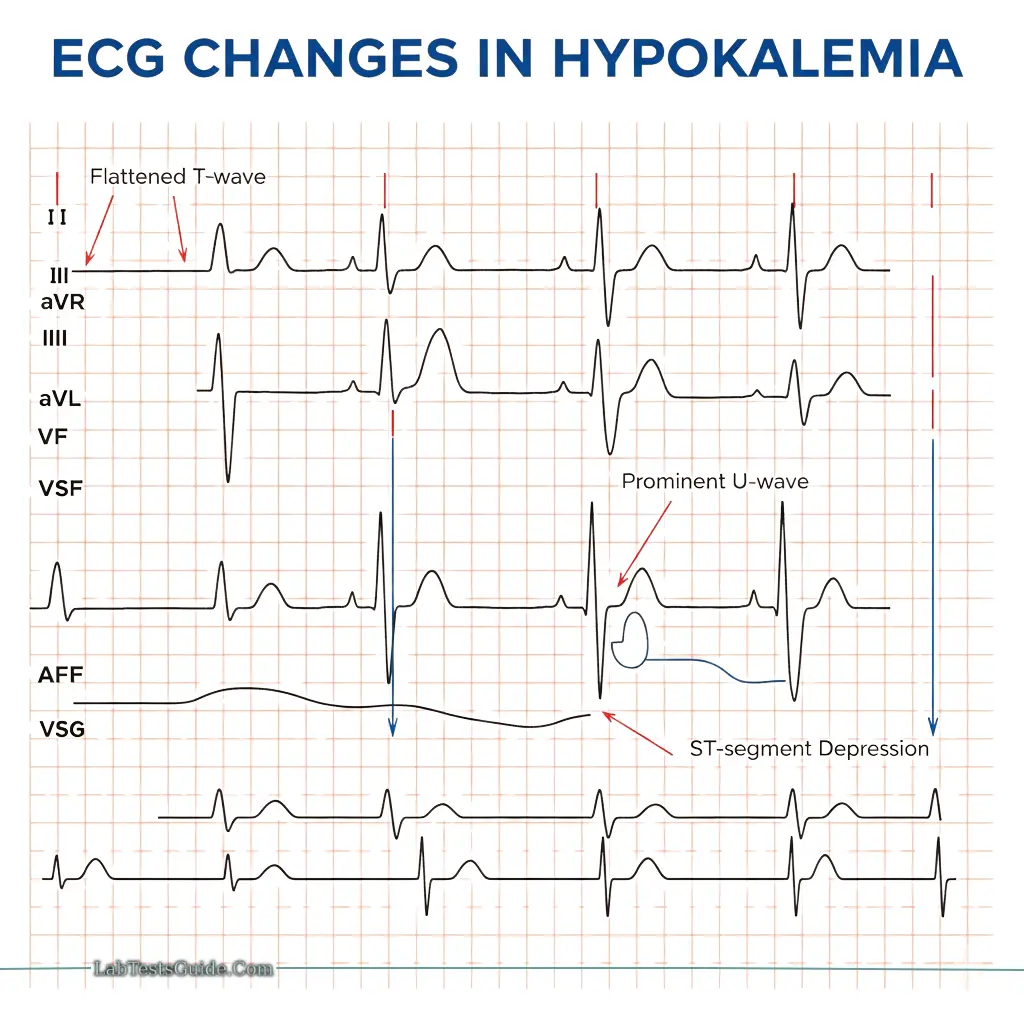
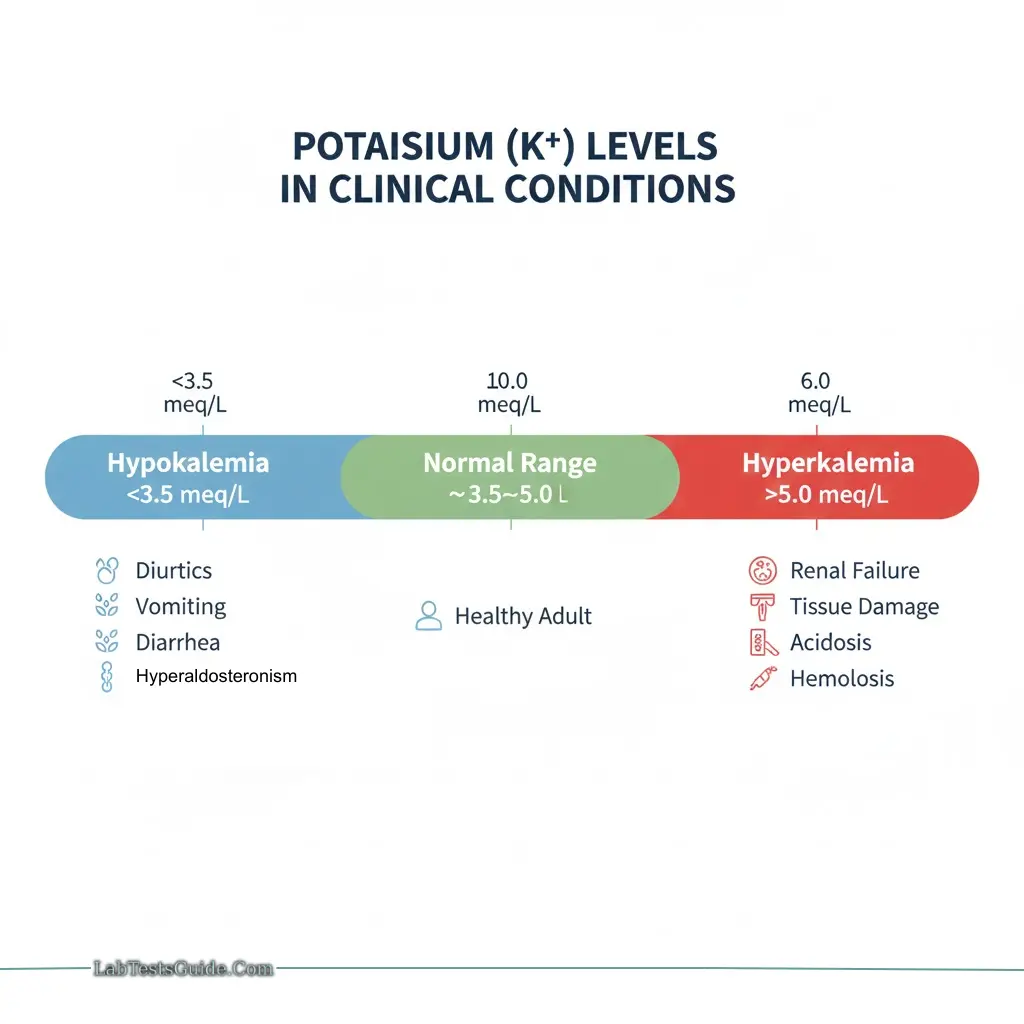
Hypokalemia:
- Causes: Diuretics, vomiting, diarrhea, hyperaldosteronism
- ECG changes: Flattened T-wave, prominent U-wave, ST depression
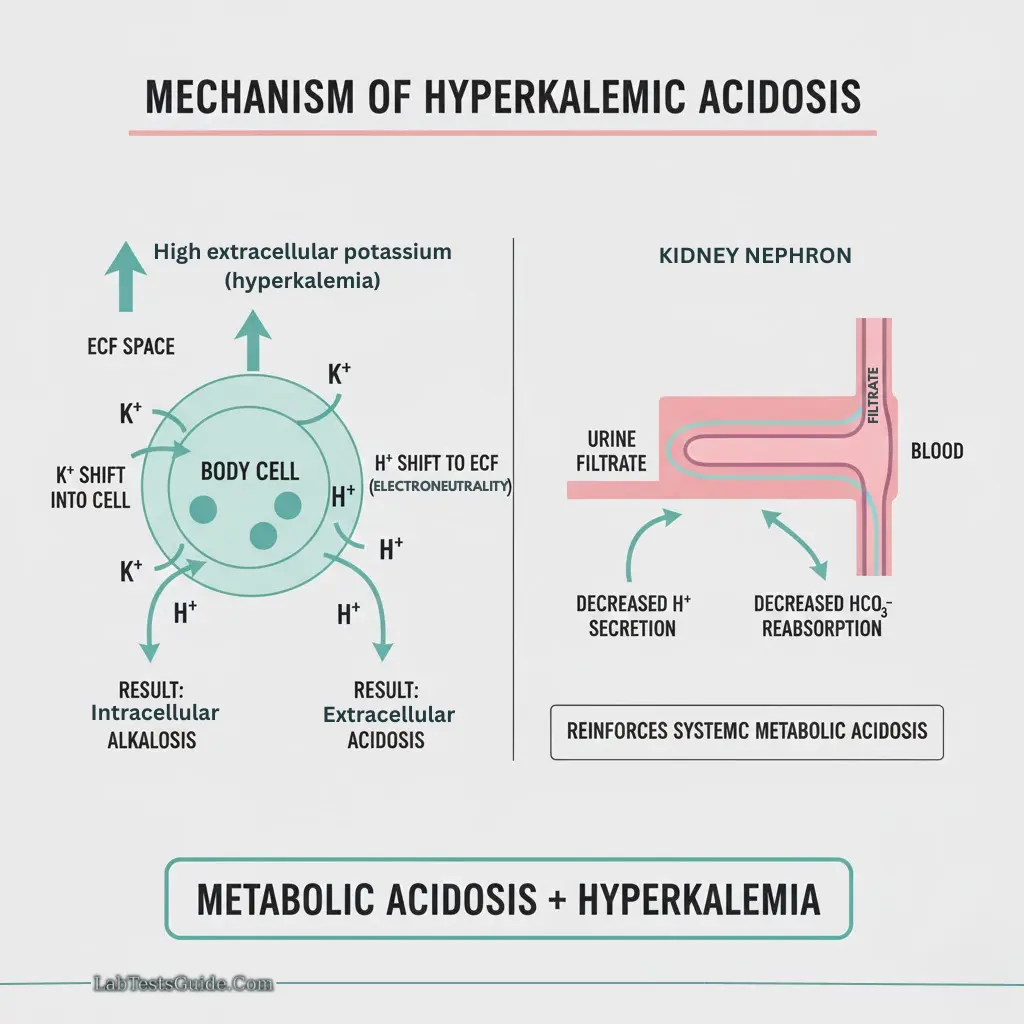
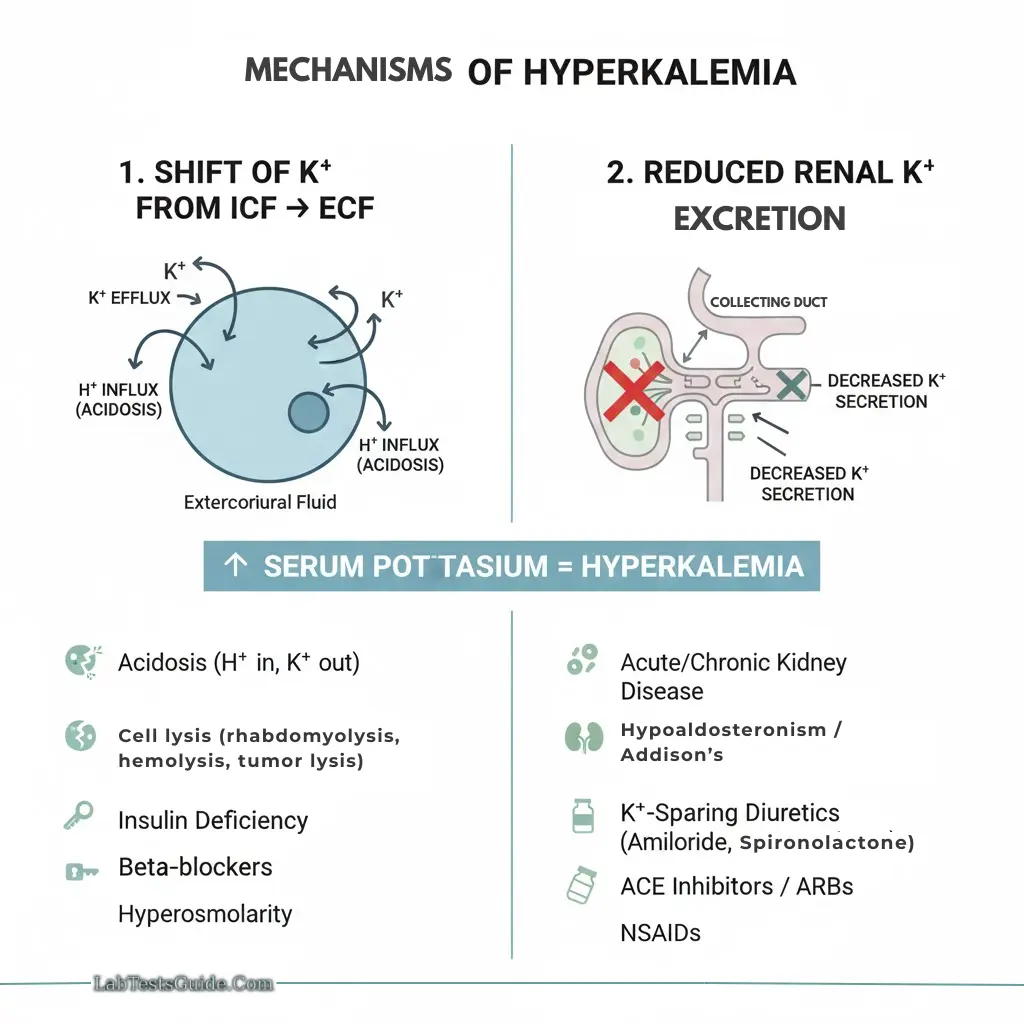
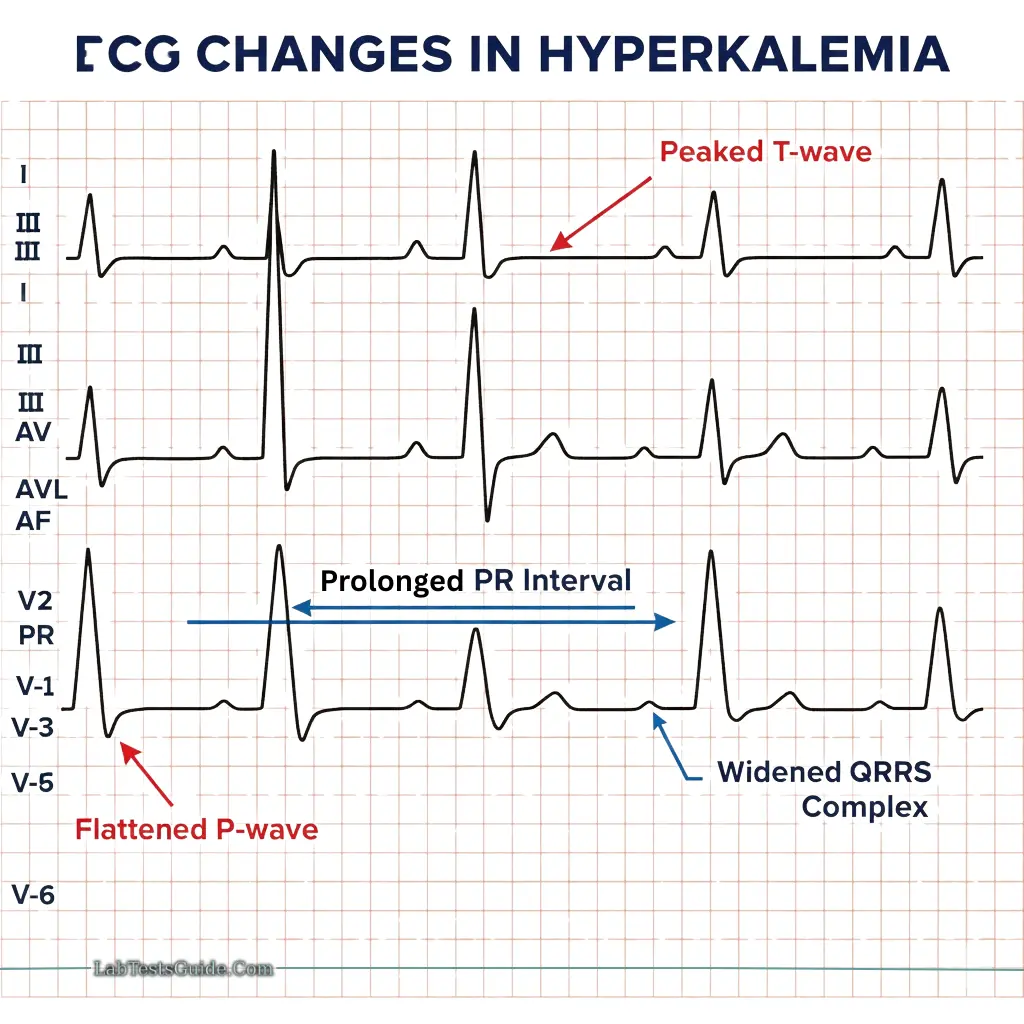
Hyperkalemia:
- Causes: Renal failure, tissue damage, hemolysis, metabolic acidosis
- ECG changes: Peaked T-wave, flattened P-wave, prolonged PR/QRS
Potassium Shifts:
- Acidemia: K⁺ moves out of cells → hyperkalemia
- Alkalemia: K⁺ moves into cells → hypokalemia
Sodium (Na⁺)
Physiology
Sodium is the major extracellular cation, crucial for plasma osmolality, fluid balance, and electric neutrality.
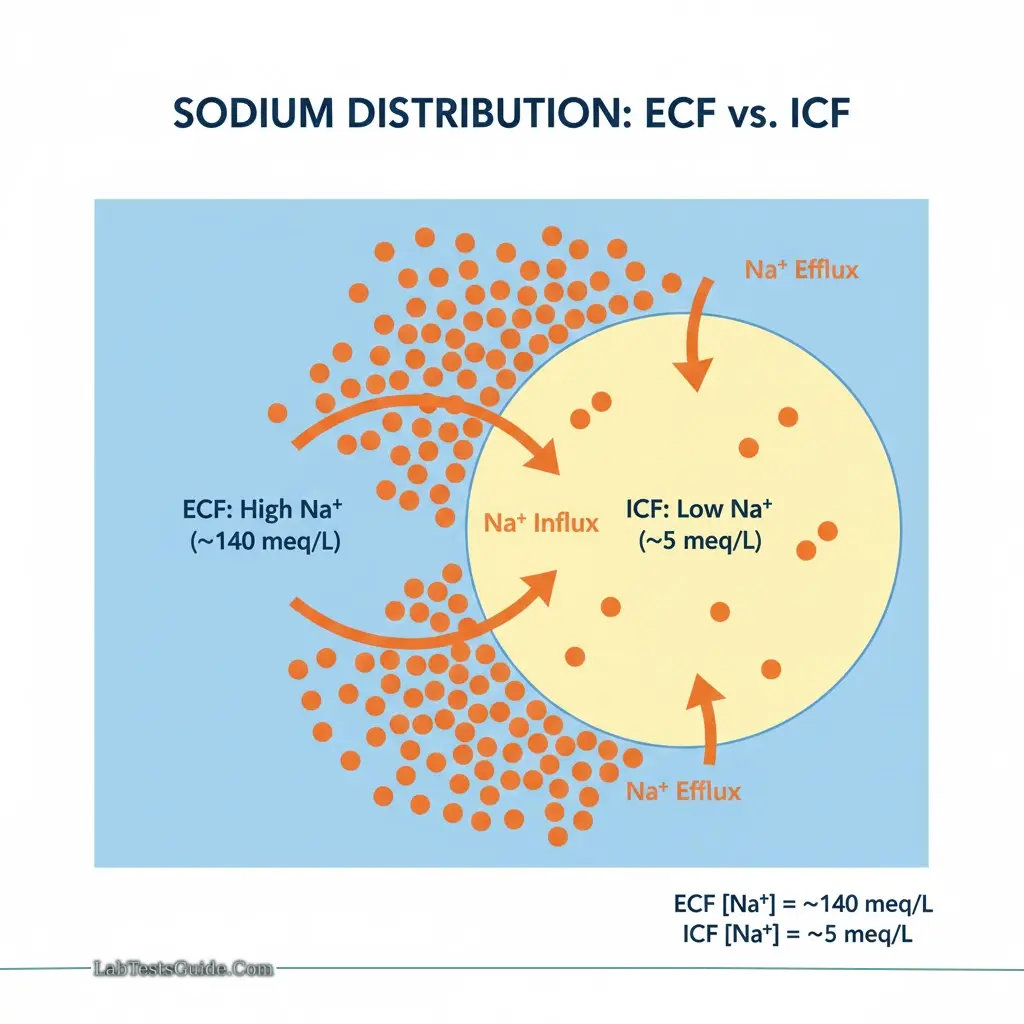
Distribution:
- Extracellular: ~140 meq/L
- Intracellular: ~5 meq/L
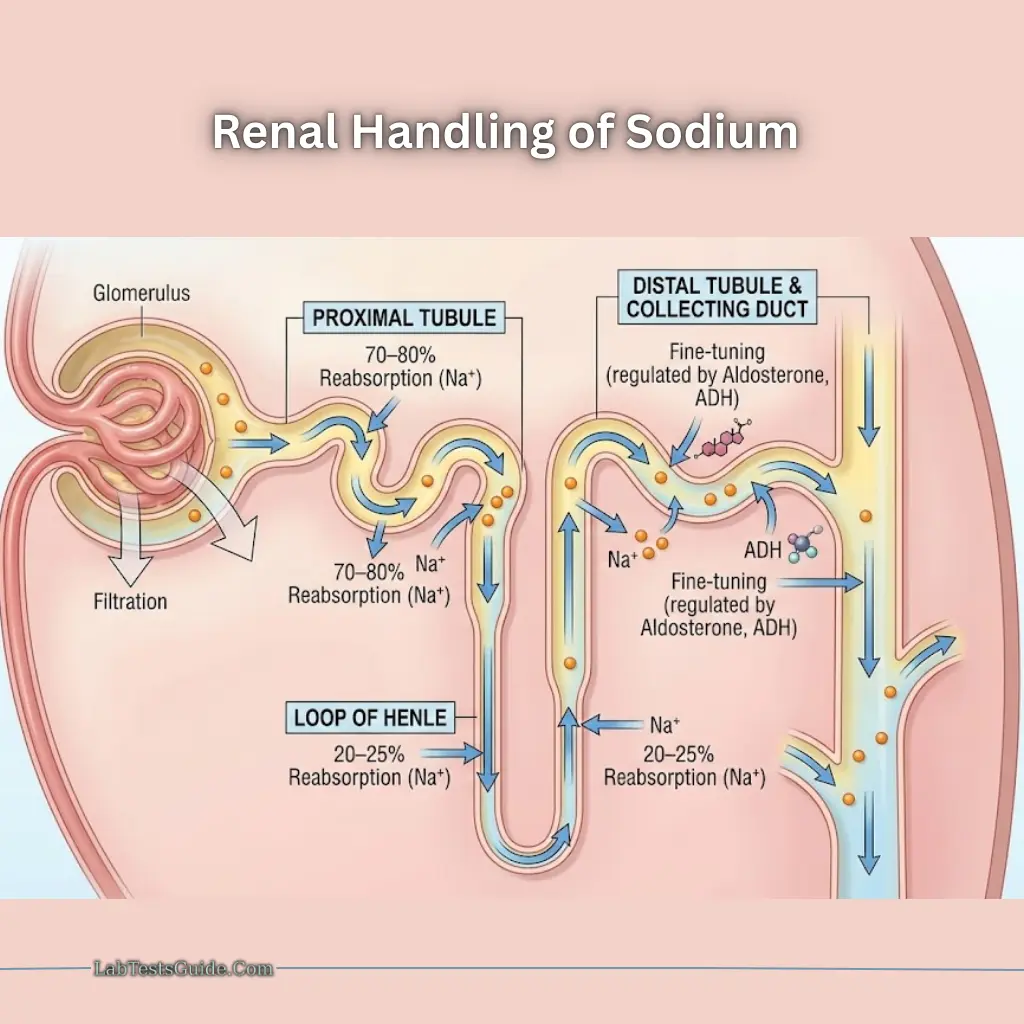
Renal Handling
- 100% filtered at glomerulus
- 70–80% reabsorbed in proximal tubules with water and chloride
- 20–25% reabsorbed in loop of Henle
- Distal nephron and collecting ducts regulate Na⁺ based on aldosterone and ADH
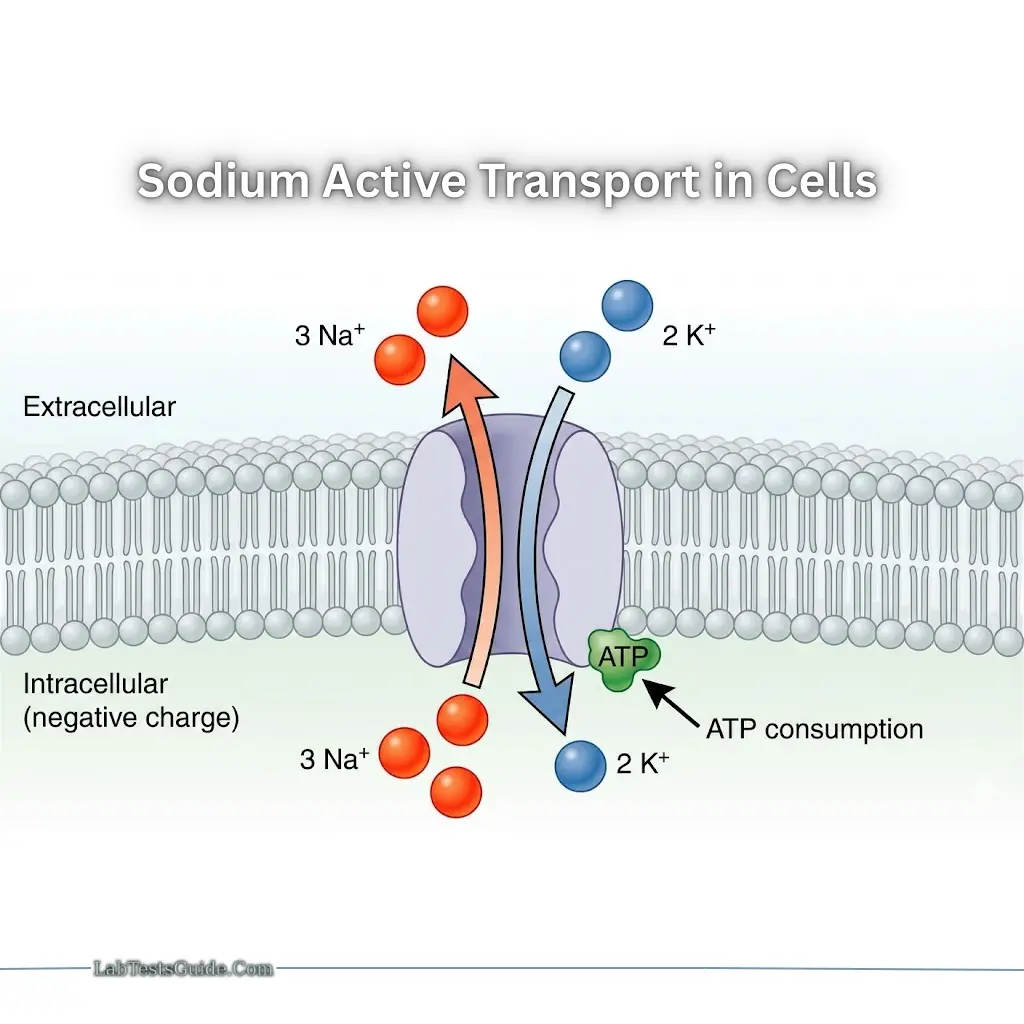
Active Transport
- Na⁺/K⁺ ATPase pump: 3 Na⁺ out, 2 K⁺ in → maintains negative intracellular charge
Functions
- Maintains extracellular osmolality and volume
- Nerve impulse transmission
- Muscle contraction with K⁺ and Ca²⁺
- Acid-base balance via sodium bicarbonate and phosphate
Clinical Significance
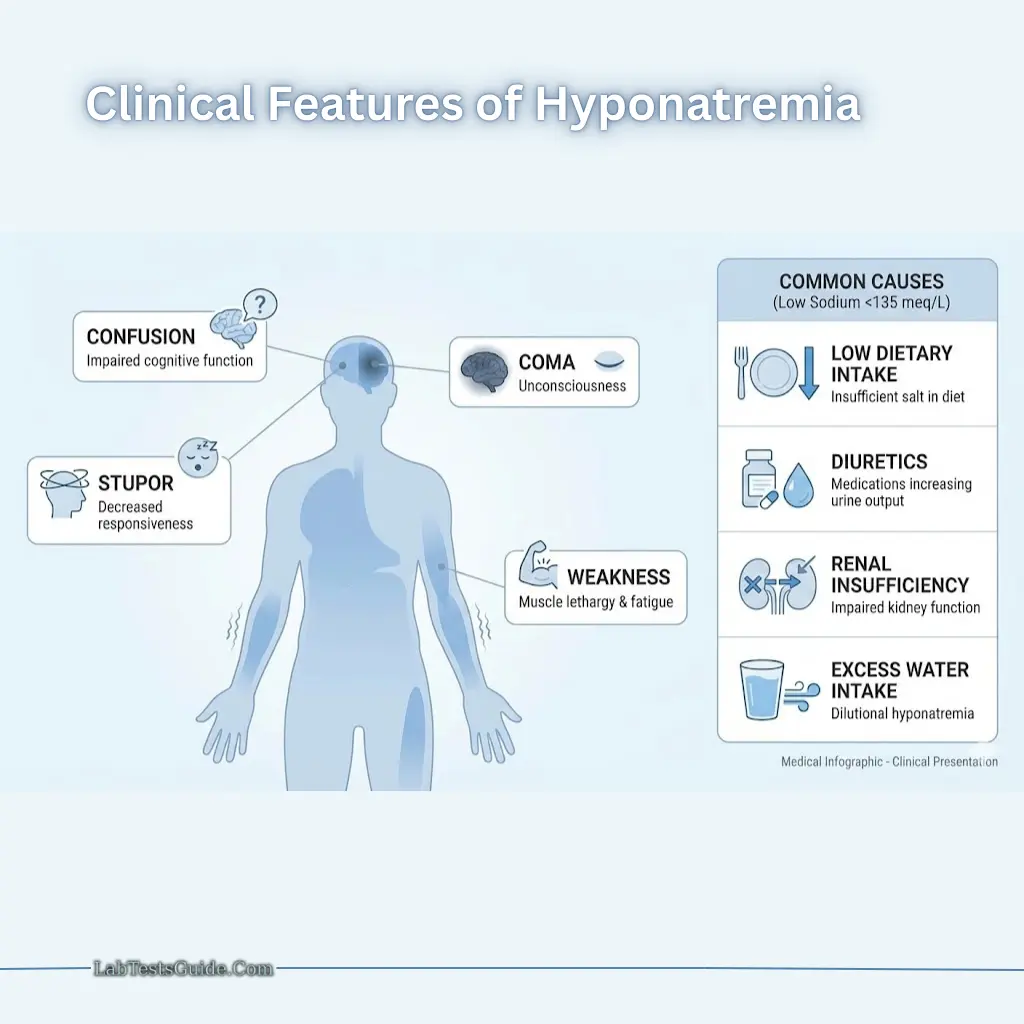
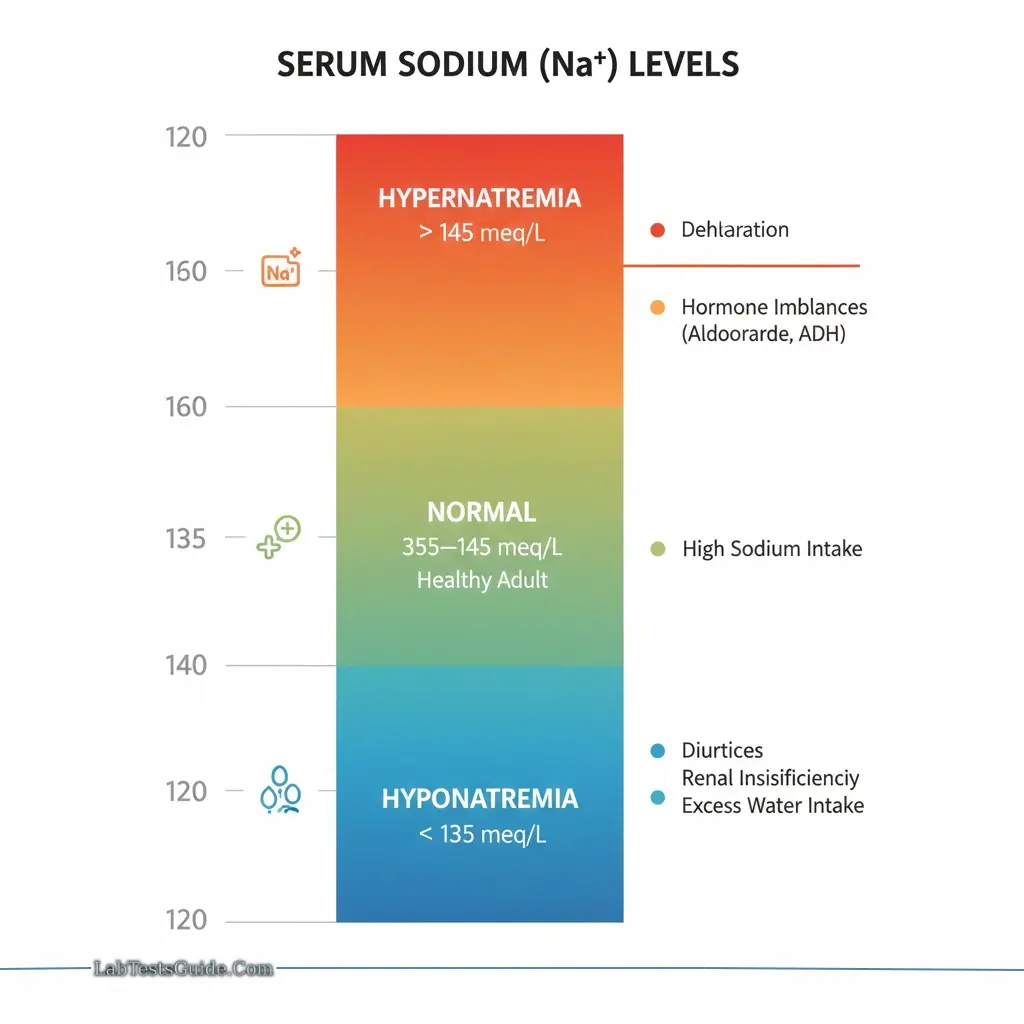
Hyponatremia (<135 meq/L):
- Symptoms: Weakness, confusion, stupor, coma
- Causes: Low dietary intake, diuretics, renal insufficiency, excess water intake
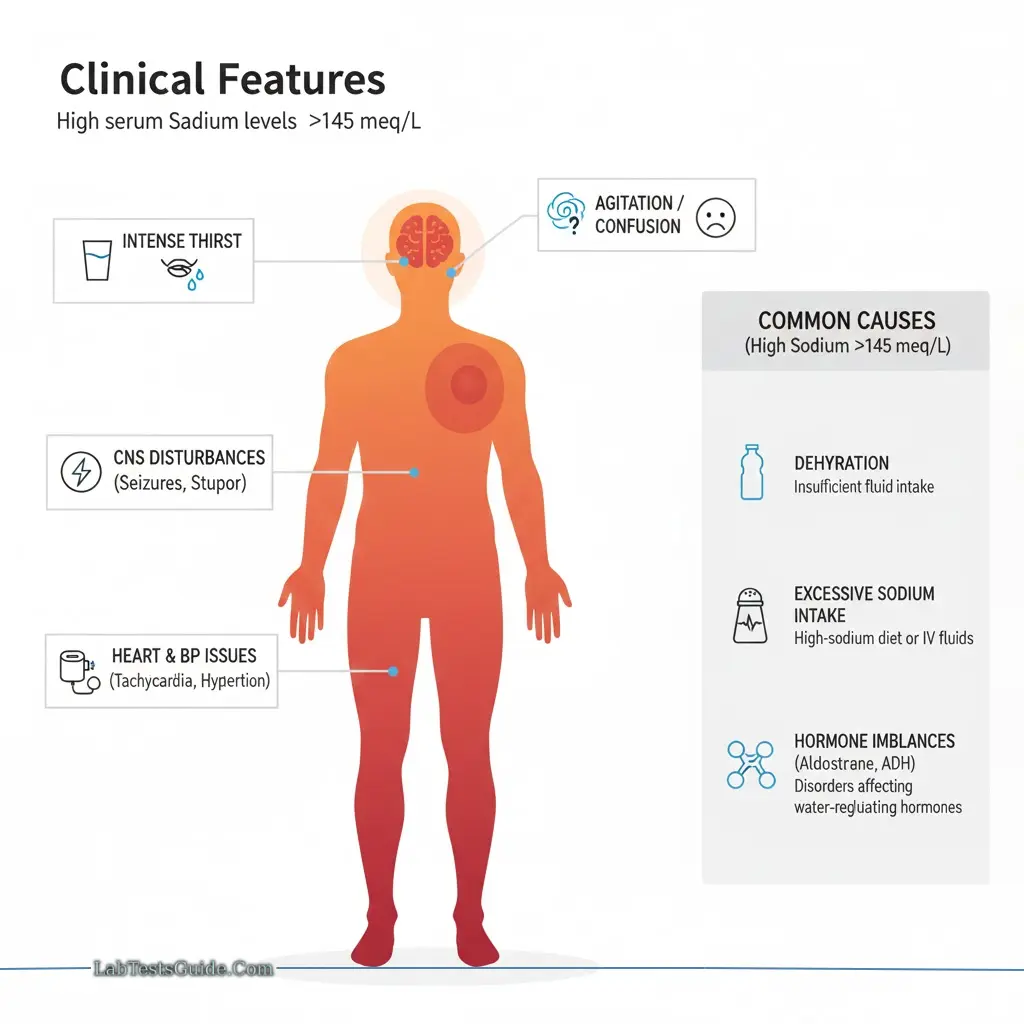
Hypernatremia (>145 meq/L):
- Symptoms: Thirst, agitation, CNS disturbances, heart failure
- Causes: Dehydration, excessive Na⁺ intake, hormone imbalances (aldosterone, ADH)
Electrolytes Panel
Definition: A set of tests measuring Na⁺, K⁺, Cl⁻, HCO₃⁻, CO₂, Ca²⁺, Mg²⁺, phosphate.
Functions:
- Maintain osmotic pressure
- Regulate heart rhythm and muscle contraction
- Support brain function and energy production
- Maintain acid-base balance and prevent dehydration
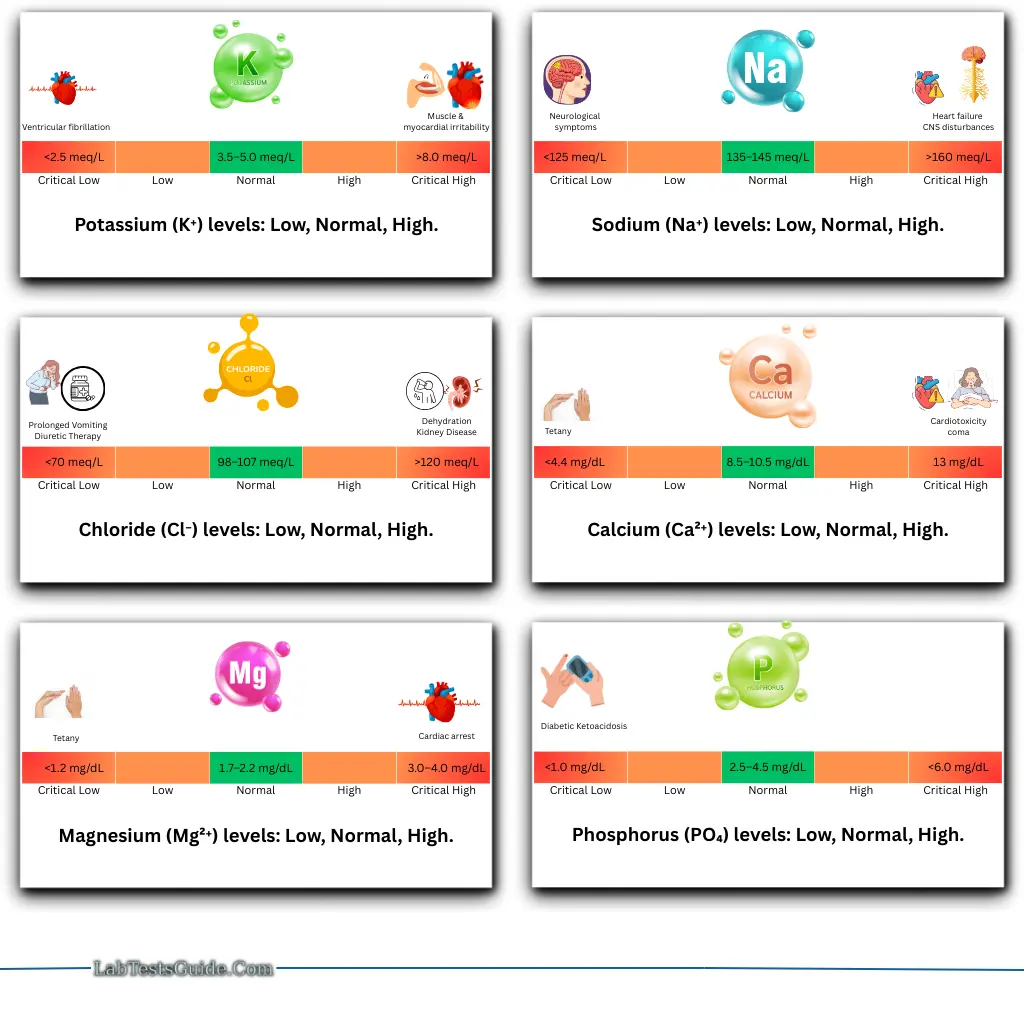
Critical Values (Examples):
| Electrolyte | Low Value | High Value |
|---|---|---|
| K⁺ | <2.5 meq/L → Ventricular fibrillation | >8.0 meq/L → Muscle & myocardial irritability |
| Na⁺ | <125 meq/L → Neurological symptoms | >160 meq/L → Heart failure |
| Ca²⁺ | <4.4 mg/dL → Tetany | >13 mg/dL → Cardiotoxicity, coma |
| Mg²⁺ | <1.2 mg/dL → Tetany | 30–40 mg/dL → Cardiac arrest |
| Cl⁻ | <70 meq/L | >120 meq/L |
| PO₄³⁻ | <1.0 mg/dL | – |
| CO₂ / HCO₃⁻ | <10 meq/L | >40 meq/L |
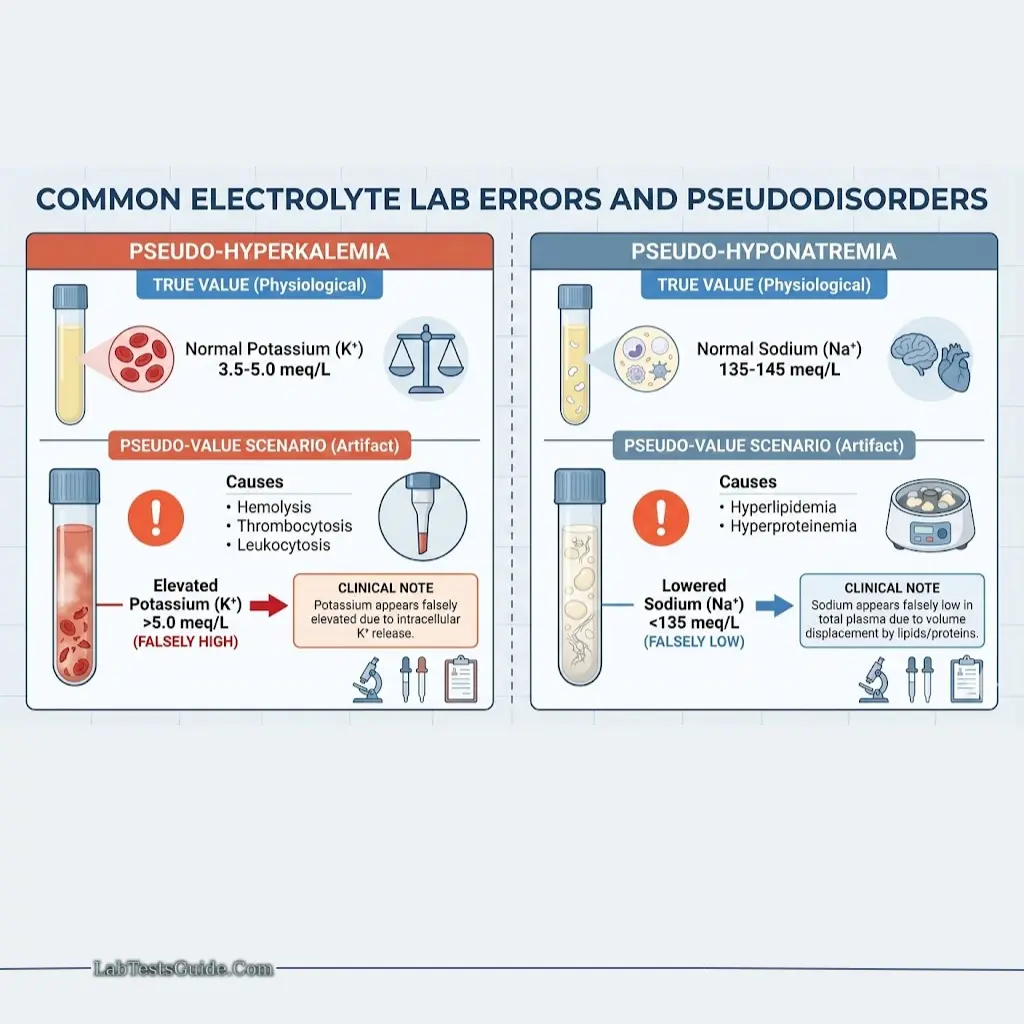
Lab Errors and Pseudodisorders:
- Pseudo-hyperkalemia: Hemolysis, thrombocytosis
- Pseudo-hyponatremia: Hyperlipidemia, hyperproteinemia
Summary / Quick Reference
- Electrolytes are essential for cell function, nerve and muscle activity, cardiac rhythm, osmotic balance, and acid-base homeostasis.
- Sodium dominates the ECF, Potassium dominates the ICF.
- Electrolyte disturbances manifest as neurological, muscular, or cardiac complications.
- Regular monitoring via an electrolyte panel is crucial in critically ill patients, those on diuretics, or patients with renal or endocrine disorders.
Understand Your Test Results:
Understand your Electrolytes Results
AI-powered Lab Test Results Meaning tool 🤖