Clinical research across Europe is shaped by a combination of shared regulatory frameworks and country-specific procedures. As studies expand across borders and therapeutic areas grow more specialized, structured operational support becomes essential. Organizations working as a CRO Europe or a CRO Poland provide regional insight and coordinated study management, helping sponsors navigate both the unified EU regulatory environment and the practical differences among individual countries.
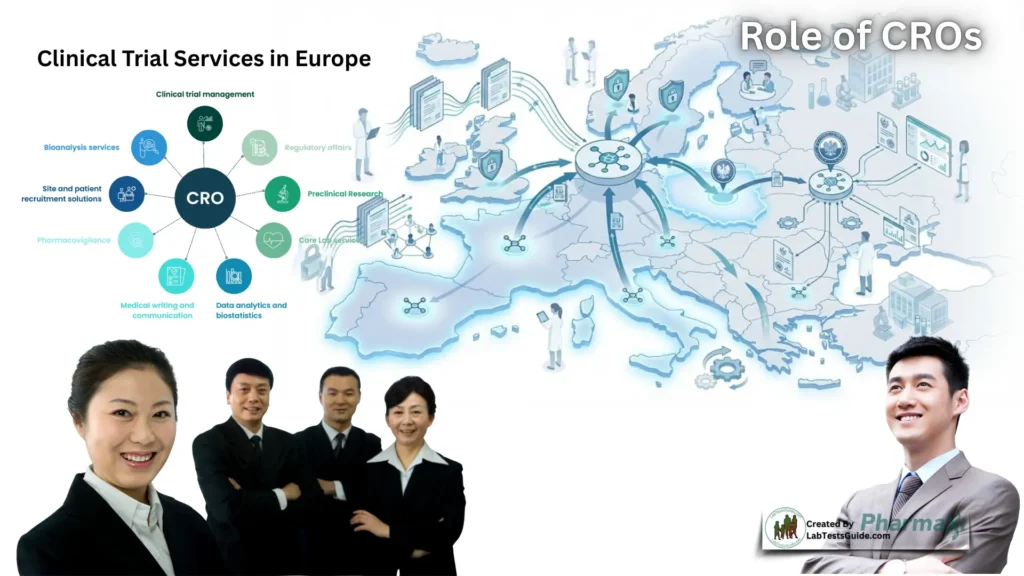
The Role of a CRO Europe
A CRO Europe supports clinical trials conducted across the continent by aligning study activities with European regulatory requirements and harmonized guidelines. Its responsibilities typically include:
- managing submissions under the EU Clinical Trials Regulation (EU CTR),
- coordinating communication with ethics committees and competent authorities in multiple countries,
- ensuring compliance with GDPR for all participant data,
- harmonizing procedures across geographically diverse sites,
- supporting multilingual documentation and investigator communication.
This regional-level coordination helps sponsors avoid administrative delays and ensures that multicenter studies follow consistent operational standards. European CROs also maintain networks of investigators and specialized research centers, which can improve feasibility assessments and recruitment planning.
The Role of a CRO Poland
Poland has become an important location for European clinical research due to its strong network of clinical sites, high investigative expertise, and efficient participant recruitment processes. A CRO Poland provides localized support that reflects national procedures and research infrastructure.
Typical responsibilities include:
- preparing and managing submissions to Polish ethics committees and regulatory authorities,
- coordinating site initiation and staff training based on national standards,
- supporting documentation in Polish and managing translations when required,
- monitoring trial conduct according to local expectations and ICH-GCP,
- advising sponsors on site capabilities in areas such as oncology, cardiology, immunology, and neurology.
Local insight is particularly valuable during study start-up, where procedural clarity and communication with authorities influence the timeline significantly.
Integrating Regional and Local Expertise
Although EU CTR provides harmonization, each European country maintains specific timelines, document requirements, and institutional processes. For this reason, many multinational studies rely on both:
- a CRO Europe for cross-country coordination, and
- local partners such as a CRO Poland for national-level operational tasks.
This combined approach supports:
- consistent monitoring strategies across regions,
- unified data management practices,
- risk-based operational oversight,
- clear escalation pathways for site issues,
- alignment with both pan-European and country-specific regulatory expectations.
Data Management and Quality Frameworks
Regardless of region, CROs must follow strict quality systems that ensure patient safety and data reliability. This includes:
- adherence to ICH-GCP,
- compliance with GDPR for all personal data,
- standardized documentation and audit trails,
- routine oversight visits or remote monitoring,
- training programs for investigators and study teams.
These frameworks help maintain the scientific validity of the study and ensure that trial results meet the expectations of regulatory authorities.
Summary
Both CRO Europe and CRO Poland play essential roles in supporting clinical trials. Regional CROs coordinate large, multi-country studies and align them with EU regulatory systems, while Polish CROs provide practical, country-specific operational expertise. Together, they contribute to consistent trial conduct, reliable data, and efficient study progression across the European research landscape.
⚠️ Disclaimer:
The content on LabTestsGuide.com is for informational and educational purposes only. We do not guarantee the accuracy, completeness, or timeliness of the information provided. Always consult qualified healthcare professionals for medical advice, diagnosis, or treatment. LabTestsGuide.com is not liable for any decisions made based on the information on this site.