Anti-Xa Activity Calculation: Laboratory Methodology
For laboratories performing heparin monitoring, Anti-Xa activity quantifies heparin concentration through chromogenic assays. This section explains the core calculation principle for lab professionals and students.

• Assay Factor: Reagent-specific calibration constant
• Anti-Xa (IU/mL): Functional heparin activity
• Therapeutic Range: 0.3-0.7 IU/mL (standard dosing)
• High-Range: 0.5-1.1 IU/mL (PCI/ECMO)
• Measures inhibition of Factor Xa
• Calibrated against WHO heparin standard
• Requires specific reagent kit
• Run in duplicate for accuracy
• Reported in international units (IU/mL)
• Assay Factor: 1.12 (kit-specific)
• Anti-Xa = 0.45 × 1.12 = 0.50 IU/mL
• Interpretation: Therapeutic range
• Action: Maintain current infusion
• Unaffected by lupus anticoagulants
• Better accuracy in pregnancy
• Preferred in obesity/critical illness
• Gold standard for LMWH monitoring
• Correlates with thrombotic risk
• 3-4 hours after infusion start/change
• Use citrate tube (light blue top)
• Process within 1 hour
• Avoid heparin-contaminated lines
• Steady-state: After 3-4 half-lives
• Not real-time (30-60 min turnaround)
• Higher cost than aPTT
• Affected by elevated bilirubin
• Less sensitive to very low heparin
• Interference from direct Xa inhibitors
• Assay factor is reagent-specific (check package insert)
• Therapeutic range may vary by clinical indication
• Results >1.0 IU/mL → high bleeding risk
• Always correlate with clinical assessment
• Consider anti-thrombin deficiency if heparin resistance
🧪 Anti-Xa Activity Calculation
📐 Formula:
🖊️ Enter Plasma Heparin Concentration (IU/mL):
Fundamental Formula
Anti-Xa Activity (IU/mL) = Plasma Heparin Concentration (IU/mL) × Heparin Anti-Xa Assay Factor
Components Explained:
- Plasma Heparin Concentration
- Measured in IU/mL (International Units per milliliter)
- Derived from patient plasma samples collected in citrate tubes
- Heparin Anti-Xa Assay Factor
- Laboratory-specific calibration constant
- Determined using WHO-standardized heparin preparations
- Validated per CLIA/CAP guidelines (typically 0.9–1.1 IU/mL per unit)
Workflow Overview
- Sample Processing:
- Centrifuge blood at 1,500–2,500 × g for 15 minutes
- Harvest platelet-poor plasma (<10,000 platelets/μL)
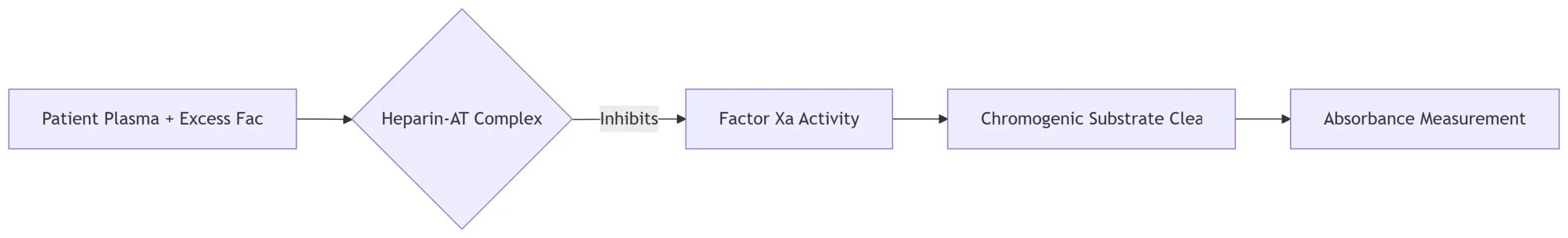
- Reaction Principle:

- Calculation Steps:
- Measure absorbance at 405 nm
- Compare against standard curve (0–1.0 IU/mL heparin)
- Apply institution-specific assay factor:
Reported Anti-Xa (IU/mL) = Observed Value × Assay Factor
Key Considerations for Lab Professionals
- Calibration:
- Run daily controls with low/mid/high heparin concentrations
- Recalibrate if QC exceeds ±15% of target
- Interferences:
- Falsely ↑: Hyperbilirubinemia (>15 mg/dL), hemolysis (>500 mg/dL Hb)
- Falsely ↓: Factor X deficiency, elevated fibrinogen
- Therapeutic Ranges:
Clinical Context Target Anti-Xa (IU/mL)
Prophylactic LMWH 0.2–0.4
Therapeutic UFH 0.3–0.7
ECMO/CPB 0.5–0.8 🔬 Best Practice: Report values with collection timestamps (e.g., “Anti-Xa: 0.45 IU/mL @ 4h post-bolus”) Clinical-Laboratory Interface- Critical Values Protocol:
- >1.0 IU/mL: Immediately call treating team (bleeding risk)
- <0.2 IU/mL: Flag for potential under-anticoagulation
- Trend Monitoring:
- Correlate with aPTT when discordant results occur
- Document heparin lot # in outbreaks of unexpected values