Test your understanding of Vitamins, Minerals, and Electrolyte Balance with this Biochemistry Mock Test (Part 51) . This practice quiz is designed for medical laboratory students and professionals preparing for the ASCP MLS and Clinical Chemistry exams. It focuses on the essential nutrients, trace elements, and electrolytes that regulate metabolic and physiological stability.
📘 Topics Included Vitamin functions and deficiency diseases Major and trace minerals in metabolism Electrolyte balance and osmoregulation Laboratory methods for electrolyte and mineral analysis Clinical interpretation in metabolic and renal disorders 🧠 Why Take This Mock Test? Reinforce your understanding of nutritional and biochemical balance. Prepare effectively for ASCP-style clinical chemistry questions. Identify weak areas in electrolyte interpretation. Build confidence in handling lab-based biochemical data.
Report a question
ASCP MLS Exam MCQs Chapter 51
Why Take This Mock Test? Strengthens exam confidence Highlights areas for improvement Provides practice with clinically relevant scenarios This mock test (60 MCQs (4000 – 4060) ) is part of our ongoing ASCP MLS Exam Practice Series , giving you structured preparation for all major immunology topics.
Our Biochemistry – Vitamins, Minerals & Electrolyte Balance Mock Test is specifically designed for candidates appearing in ASCP MLS, AMT MLT/MT, AIMS, CSMLS, IBMS, HAAD/DOH, DHA, and MOH exams. This mock test mirrors the structure, difficulty level, and question style you can expect in the actual examination.
Take this test to:ASCP MLS Exam .
Who Should Use This Mock Test? Medical Laboratory Scientists and Technicians
Pathology Students
Professionals preparing for international laboratory certification exams
Anyone seeking to strengthen their knowledge of Biochemistry – Vitamins, Minerals & Electrolyte Balance
How to Use This Mock Test Effectively Simulate Exam Conditions: Attempt the test in one sitting without referring to notes.
Track Your Time: Keep within the allotted time limit to build speed.
Review Explanations: Study the answer explanations to strengthen understanding.
Repeat for Retention: Re-attempt after revision to measure improvement.
1 / 60
Category:
ASCP Exam Questions
Which percentage of total serum calcium is bound to protein (primarily albumin) and is thus nondiffusible?
Total serum calcium exists in three forms:
Protein-bound (∼40-50%): This fraction is primarily bound to albumin (about 80% of the bound fraction) and, to a lesser extent, globulins. This form is non-diffusible and thus physiologically inactive.
Ionized (∼45-50%): This is the free, physiologically active form that is tightly regulated by parathyroid hormone (PTH). It is diffusible and responsible for functions like neuromuscular conduction and blood coagulation.
Complexed (∼5-10%): This fraction is bound to anions like citrate, phosphate, and bicarbonate. It is diffusible but not ionically active.
2 / 60
Category:
ASCP Exam Questions
Pellagra is caused by a deficiency of:
Other options:
a) Thiamine (B1) → beriberi, Wernicke-Korsakoff syndrome
c) Riboflavin (B2) → ariboflavinosis (cheilosis, glossitis)
d) Biotin (B7) → dermatitis, alopecia, neurological symptoms
3 / 60
Category:
ASCP Exam Questions
Iron in the blood is primarily bound to:
Iron in the blood is transported by the protein transferrin . It binds to iron absorbed from the diet or released from storage and delivers it to tissues, particularly the bone marrow for hemoglobin synthesis.
Why the other options are incorrect:
a) Albumin: This protein transports many substances (like fatty acids and hormones), but it is not the primary carrier for iron.
b) Hemoglobin: This is the iron-containing protein inside red blood cells that carries oxygen. It is the major user of iron in the body, but it is not the transport protein in the blood.
d) Ferritin: This is the primary intracellular iron-storage protein, found in the liver, spleen, and bone marrow. A small amount of ferritin circulates in the blood as a marker of iron stores, but it does not function as a transport protein.
4 / 60
Category:
ASCP Exam Questions
The solute that contributes the most to the total serum osmolality is:
Serum osmolality is a measure of the concentration of dissolved particles in the blood. The formula for calculated serum osmolality highlights which solutes contribute most:
Calculated Osmolality = 2[Na⁺] + [Glucose]/18 + [BUN]/2.8
Sodium (Na⁺) and its associated anions (like chloride and bicarbonate) account for approximately 90-95% of the total serum osmolality. This is because sodium is by far the most abundant solute in the extracellular fluid, and the “2” in the formula accounts for both the sodium cation and its accompanying anions.
Why the other options are incorrect:
a) Glucose: While an important osmole, its normal concentration (~70-100 mg/dL) contributes very little compared to sodium (~140 mEq/L). It only becomes a significant contributor in extreme hyperglycemia.
b) Urea: Urea is a passive osmole that crosses cell membranes easily and therefore contributes less to the effective osmolality (tonicity). Its normal concentration contributes a relatively small portion to the total.
c) Chloride: While a major anion, it is paired with sodium. Sodium is the primary driver, and chloride’s concentration generally follows that of sodium.
5 / 60
Category:
ASCP Exam Questions
A hospitalized patient experiences tetany (increased neuromuscular irritability). Which test should be ordered immediately?
Tetany (muscle cramps, spasms, or carpopedal spasms) is a classic sign of hypocalcemia .
Immediate measurement of serum calcium is essential to confirm the diagnosis and guide urgent treatment.
Other options:
a) Phosphate → may be relevant later; high phosphate can contribute to hypocalcemia, but it is not the first test .
b) BUN → evaluates kidney function, not directly related to tetany.
c) Glucose → hypoglycemia can cause neurological symptoms but tetany is more specific for calcium imbalance .
6 / 60
Category:
ASCP Exam Questions
A reciprocal relationship exists between the serum concentrations of:
A reciprocal (inverse) relationship exists between the serum concentrations of calcium and phosphate (inorganic phosphorus) . This relationship is primarily regulated by parathyroid hormone (PTH) and vitamin D to maintain the solubility product of calcium and phosphate, which is critical for bone mineralization and to prevent the precipitation of calcium phosphate crystals in soft tissues.
Why the other options are incorrect:
a) Sodium and Potassium: These electrolytes are regulated independently by different mechanisms (e.g., aldosterone affects both but does not create a strict reciprocal relationship).
c) Chloride and Bicarbonate: These anions often shift in relation to acid-base status (e.g., a rise in chloride is associated with a fall in bicarbonate in metabolic acidosis), but this is not a direct hormonal reciprocal relationship like that of calcium and phosphate.
d) Calcium and Magnesium: These cations can influence each other (e.g., severe hypomagnesemia can impair PTH secretion), but they do not have a direct, constant reciprocal relationship.
7 / 60
Category:
ASCP Exam Questions
Assay of transketolase activity in red blood cells is used to detect a deficiency of:
Transketolase is an enzyme in the pentose phosphate pathway. Its activity is dependent on thiamine pyrophosphate (TPP) , which is the active coenzyme form of thiamine (Vitamin B1) .
In a thiamine deficiency, the transketolase enzyme has reduced activity.
The assay measures transketolase activity in red blood cells before and after the addition of TPP.
A significant increase in activity after TPP addition (known as the TPP effect) is a specific and sensitive indicator of thiamine deficiency.
This test is considered a functional assessment of thiamine status.
Why the other options are incorrect:
b) Folic Acid: Deficiency is assessed by measuring serum or red blood cell folate levels.
c) Ascorbic Acid (Vitamin C): Deficiency is assessed by measuring serum ascorbate levels or via clinical signs of scurvy.
d) Riboflavin (Vitamin B2): Deficiency is often assessed by measuring the activity of the erythrocyte glutathione reductase activation coefficient (EGRAC), which is dependent on riboflavin.
8 / 60
Category:
ASCP Exam Questions
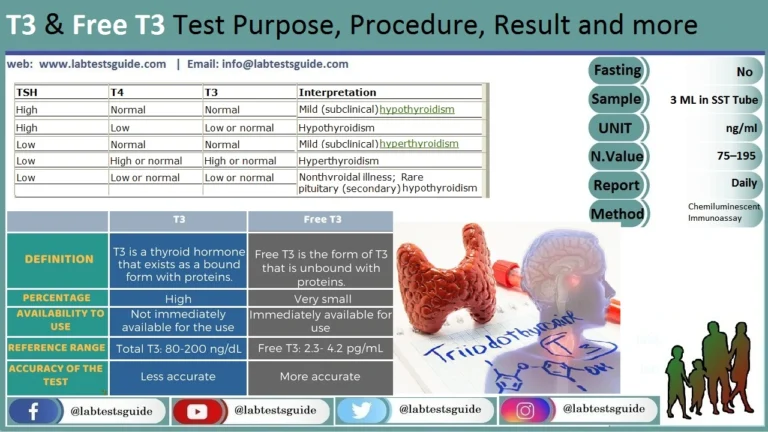
The trace element essential for thyroid hormone synthesis is:
Iodine is an essential trace element that is a fundamental component of the thyroid hormones thyroxine (T4) and triiodothyronine (T3) . The thyroid gland actively traps iodide from the blood and incorporates it into the structure of these hormones. A deficiency of iodine leads to decreased production of thyroid hormones, which can cause goiter and hypothyroidism.
Why the other options are incorrect:
a) Zinc: This is a cofactor for many enzymes (e.g., carbonic anhydrase, alkaline phosphatase) but is not involved in thyroid hormone synthesis.
c) Copper: This is essential for iron metabolism and is a cofactor in enzymes like cytochrome c oxidase and superoxide dismutase, but not for thyroid hormones.
d) Manganese: This is a cofactor for enzymes such as arginase and pyruvate carboxylase, but it is not required for thyroid hormone production.
9 / 60
Category:
ASCP Exam Questions
A potassium level of 6.8 mEq/L is obtained. Before reporting, the technologist should first:
Hyperkalemia (high potassium) can be artifactual if the blood sample is hemolyzed , because red blood cells contain high potassium .
Before reporting a critically high potassium result (6.8 mEq/L), the technologist should:
Examine the specimen for hemolysis (pink/red discoloration).
If hemolysis is present, repeat the test on a fresh specimen .
Other options:
a) Rerun the test on the same specimen → may repeat the artifact if hemolysis is the cause.
c) Check the age of the patient → irrelevant for acute potassium verification.
d) Report the result immediately → could lead to unnecessary or harmful intervention if the result is spurious.
10 / 60
Category:
ASCP Exam Questions
Rickets in children is associated with a deficiency of which vitamin?
Rickets is a childhood bone disorder characterized by soft, weak, and deformed bones. It is caused primarily by a deficiency of vitamin D .
Vitamin D is essential for the absorption of calcium and phosphorus from the intestines.
A deficiency leads to inadequate mineralization of the growing bones, resulting in the classic skeletal deformities seen in rickets (such as bowed legs, knobby projections on the ribs, and a pigeon chest).
Why the other options are incorrect:
a) Vitamin B1 (Thiamine): Deficiency causes beriberi.
b) Vitamin C: Deficiency causes scurvy.
c) Niacin (Vitamin B3): Deficiency causes pellagra.
11 / 60
Category:
ASCP Exam Questions
Which of the following electrolytes is the chief plasma cation whose main function is maintaining osmotic pressure?
Sodium (Na⁺) is the most abundant cation in the extracellular fluid, including plasma.
Its primary function is to regulate and maintain osmotic pressure and water balance between body compartments. Where sodium goes, water follows.
It also plays critical roles in nerve impulse conduction and muscle contraction.
Why the other options are incorrect:
a) Chloride (Cl⁻): This is the major anion in the extracellular fluid. It helps maintain electrical neutrality and is involved in acid-base balance, but it is not the chief cation .
b) Calcium (Ca²⁺): Its primary functions are in bone formation, blood coagulation, nerve transmission, and muscle contraction—not primarily osmotic pressure.
c) Potassium (K⁺): This is the chief intracellular cation. Its main roles involve maintaining resting membrane potential and cellular enzyme functions.
12 / 60
Category:
ASCP Exam Questions
Serum concentrations of vitamin B12 are typically elevated in:
Vitamin B12 (cobalamin) circulates bound to transcobalamins .
Serum B12 is typically elevated in conditions with increased production of cobalamin-binding proteins , such as:
Other options:
a) Pernicious anemia → B12 deficiency → low serum B12
b) Chronic hemodialysis → may cause B12 loss or normal levels; not typically elevated
d) Hodgkin disease → usually normal or low B12; elevation is uncommon
13 / 60
Category:
ASCP Exam Questions
The normal reference range for serum sodium is approximately:
The normal reference range for serum sodium in adults is typically 135–145 millimoles per liter (mmol/L) . This tightly regulated range is crucial for maintaining normal osmotic pressure, nerve conduction, and muscle function.
Why the other options are incorrect:
a) 90–110 mmol/L: This range is far too low and would represent severe, life-threatening hyponatremia.
c) 120–130 mmol/L: This range represents moderate to severe hyponatremia.
d) 150–160 mmol/L: This range represents hypernatremia.
14 / 60
Category:
ASCP Exam Questions
The major function of Vitamin A is related to:
Vitamin A (retinol) has two primary, critical functions:
Vision: It is a component of rhodopsin , the photopigment in the rods of the retina essential for vision in low-light conditions (scotopic vision). A deficiency leads to night blindness.
Epithelial Tissue Maintenance: It is crucial for the normal growth and differentiation of epithelial cells throughout the body, including the skin and the linings of the respiratory, gastrointestinal, and urinary tracts. It helps maintain healthy mucous barriers.
Why the other options are incorrect:
b) Collagen synthesis: This is the primary role of Vitamin C .
c) Blood coagulation: This is the primary role of Vitamin K .
d) Calcium absorption: This is the primary role of Vitamin D .
15 / 60
Category:
ASCP Exam Questions
Sodium determination by an indirect ion-selective electrode can be falsely decreased by:
Indirect ion-selective electrode (ISE) methods measure electrolytes in plasma after dilution .
Hyperlipidemia or hyperproteinemia increases the non-aqueous fraction of plasma.
Since the electrode measures the diluted plasma assuming a normal water fraction, the apparent sodium concentration is falsely decreased — this is called pseudohyponatremia .
Other options:
a) Elevated chloride levels → does not falsely decrease sodium by ISE.
c) Decreased protein levels → would have minimal effect.
d) Decreased albumin levels → mostly affects total calcium, not sodium.
16 / 60
Category:
ASCP Exam Questions
The most abundant mineral in the human body is:
Calcium is the most abundant mineral in the human body. The vast majority (about 99%) is stored in bones and teeth, providing structural strength. The remaining 1% circulates in the blood and is crucial for vital functions such as muscle contraction, nerve transmission, and blood clotting.
Why the other options are incorrect:
a) Iron: While essential for oxygen transport in hemoglobin, it is present in much smaller quantities.
b) Sodium: A major extracellular electrolyte, but not the most abundant mineral overall.
d) Potassium: The major intracellular cation, but its total body content is less than that of calcium.
17 / 60
Category:
ASCP Exam Questions
The “anion gap” is most useful as a quality control check for which set of electrolytes?
AST, ALT, LD, Amylase
BUN, Creatinine, Uric Acid
Calcium, Phosphorus, Magnesium
Sodium, Potassium, Chloride, Bicarbonate
Anion Gap=(Na⁺+K⁺)−(Cl⁻+HCO₃⁻)\text{Anion Gap} = (\text{Na⁺} + \text{K⁺}) – (\text{Cl⁻} + \text{HCO₃⁻}) Anion Gap = ( Na⁺ + K⁺ ) − ( Cl⁻ + HCO₃⁻ )
Other options:
a) Calcium, Phosphorus, Magnesium → not used for anion gap.
c) AST, ALT, LD, Amylase → liver and pancreatic enzymes; unrelated.
d) BUN, Creatinine, Uric Acid → kidney function markers; unrelated.
18 / 60
Category:
ASCP Exam Questions
Vitamin E primarily functions as a:
The primary and most well-defined function of Vitamin E (a family of compounds called tocopherols and tocotrienols) is to act as a potent, fat-soluble antioxidant .
It protects cell membranes from oxidative damage by scavenging free radicals and interrupting lipid peroxidation chain reactions.
This antioxidant activity is crucial for maintaining the integrity of cellular and subcellular membranes.
Why the other options are incorrect:
a) Coenzyme in oxidation-reduction reactions: This describes the function of many B-vitamins (like niacin and riboflavin), not Vitamin E.
b) Hormone precursor: This is the role of Vitamin D (precursor to calcitriol) and Cholesterol (precursor to steroid hormones).
d) Hemoglobin stabilizer: While Vitamin E’s antioxidant role may help protect red blood cells from oxidative stress, its primary function is not specifically to stabilize hemoglobin.
19 / 60
Category:
ASCP Exam Questions
Vitamins are best described as:
Vitamins are defined as organic compounds that are essential for normal growth, reproduction, and maintenance of health. They are required in very small amounts (micronutrients) in the diet because the body cannot synthesize them in sufficient quantities. They primarily function as coenzymes or hormones in crucial metabolic pathways.
Why the other options are incorrect:
a) Energy-producing nutrients: Vitamins themselves do not provide energy (calories). Instead, they help the body metabolize energy-producing nutrients like carbohydrates, fats, and proteins.
c) Inorganic elements needed for enzyme function: This describes minerals , not vitamins. Vitamins are organic, while minerals are inorganic.
d) Nonessential dietary components: Vitamins are essential , meaning they must be obtained from the diet as the body cannot make them (or cannot make enough of them).
20 / 60
Category:
ASCP Exam Questions
Which of the following is a fat-soluble vitamin?
Vitamins are classified into two groups based on their solubility:
Fat-Soluble Vitamins: These dissolve in fats and oils and can be stored in the body’s liver and fatty tissues. They include Vitamins A, D, E, and K .
Water-Soluble Vitamins: These dissolve in water and are generally not stored in the body. They include Vitamin C and the B-complex vitamins (such as B12 and folic acid).
Why the other options are incorrect:
a) Vitamin C: This is a water-soluble vitamin.
b) Vitamin B12: This is a water-soluble vitamin (part of the B-complex).
d) Folic acid (Vitamin B9): This is a water-soluble vitamin.
21 / 60
Category:
ASCP Exam Questions
Pernicious anemia results from deficiency of:
Pernicious anemia is a specific type of megaloblastic anemia caused by a deficiency of vitamin B12 (cobalamin) . The deficiency is not due to a lack of B12 in the diet, but rather to the malabsorption of the vitamin.
This malabsorption occurs because of a lack of intrinsic factor , a protein secreted by the parietal cells of the stomach. Intrinsic factor is essential for the absorption of vitamin B12 in the small intestine. In pernicious anemia, the body’s immune system attacks and destroys these parietal cells.
Why the other options are incorrect:
b) Vitamin D: Deficiency causes rickets or osteomalacia.
c) Vitamin A: Deficiency causes night blindness and xerophthalmia.
d) Folic acid: Deficiency causes a megaloblastic anemia, but it is not called “pernicious anemia.” Pernicious anemia is specifically linked to B12 malabsorption due to a lack of intrinsic factor.
22 / 60
Category:
ASCP Exam Questions
Calcium homeostasis is regulated by:
Other options:
a) Insulin and glucagon → regulate glucose metabolism, not calcium
c) Aldosterone and cortisol → regulate sodium, potassium, and stress response
d) Epinephrine → regulates fight-or-flight response
23 / 60
Category:
ASCP Exam Questions
Total Iron-Binding Capacity (TIBC) is a measure of the serum iron transporting capacity of:
TIBC (Total Iron-Binding Capacity) reflects the maximum amount of iron that serum proteins can bind .
The main iron-binding protein in plasma is transferrin .
Therefore, TIBC is an indirect measure of transferrin concentration and its capacity to transport iron .
Other options:
a) Hemoglobin → iron in red blood cells, not measured by TIBC.
b) Ceruloplasmin → copper-carrying protein, not related to TIBC.
d) Ferritin → intracellular iron storage protein; serum ferritin reflects iron stores , not transport capacity.
24 / 60
Category:
ASCP Exam Questions
Which of the following is the biologically active precursor of Vitamin A?
Retinol is one of the primary, preformed, and biologically active forms of Vitamin A . It is found in animal products like liver, eggs, and dairy.
Why the other options are incorrect:
a) Biotin: This is Vitamin B7, which is a cofactor for carboxylase enzymes.
c) Folic Acid: This is Vitamin B9, which is essential for DNA synthesis and cell division.
d) Ascorbic Acid: This is Vitamin C, which is a water-soluble antioxidant.
25 / 60
Category:
ASCP Exam Questions
Magnesium carbonate is added during the determination of Total Iron-Binding Capacity (TIBC) in order to:
TIBC (Total Iron-Binding Capacity) measures the maximum amount of iron that transferrin can bind .
During the assay:
Excess iron is added to saturate transferrin.
Unbound iron must be removed so only iron bound to transferrin is measured.
Magnesium carbonate acts as a precipitating agent , removing excess unbound iron from the solution.
Other options:
a) Allow the color to develop → not the role of MgCO₃
b) Precipitate protein → some methods use other reagents for protein removal
c) Bind with hemoglobin iron → not relevant; TIBC is a serum assay
26 / 60
Category:
ASCP Exam Questions
A patient has a serum iron of 250 µg/dL and a TIBC of 250 µg/dL. These results are most indicative of:
Transferrin saturation=Serum IronTIBC×100=250250×100=100%\text{Transferrin saturation} = \frac{\text{Serum Iron}}{\text{TIBC}} \times 100 = \frac{250}{250} \times 100 = 100\% Transferrin saturation = TIBC Serum Iron × 100 = 250250 × 100 = 100%
Other options:
a) Normal iron status → serum iron and TIBC would be within normal ranges.
b) Iron deficiency anemia → low serum iron, high TIBC.
c) Anemia of chronic disease → low serum iron, low TIBC.
28 / 60
Category:
ASCP Exam Questions
Which of the following minerals is a component of hemoglobin?
Iron (Fe) is an essential component of the heme group in hemoglobin. Each hemoglobin molecule contains four heme groups, and each heme group has an iron atom at its center. This iron atom is what binds to oxygen (O₂) in the lungs and releases it in the tissues, enabling red blood cells to carry oxygen throughout the body.
Why the other options are incorrect:
a) Zinc: Zinc is a cofactor for many enzymes (e.g., carbonic anhydrase) but is not part of hemoglobin.
b) Copper: Copper is involved in iron metabolism (e.g., in ceruloplasmin) and is a component of some enzymes (e.g., cytochrome c oxidase), but it is not a structural part of hemoglobin.
d) Calcium: Calcium is essential for bone health, muscle contraction, and nerve signaling, but it is not found in hemoglobin.
29 / 60
Category:
ASCP Exam Questions
In an atomic absorption method for calcium determination, lanthanum is added to the reagent to:
In atomic absorption spectroscopy (AAS) for calcium determination, phosphate ions in the sample can form insoluble complexes with calcium , reducing its availability for measurement.
Lanthanum (La³⁺) is added because it binds phosphate , preventing it from interfering with calcium absorption measurements.
This ensures accurate determination of free calcium in the sample.
Other options:
a) Internal standard → used in some spectroscopic methods, but not the primary role of lanthanum here.
b) Bind calcium for detection → lanthanum does not bind calcium for measurement; it binds phosphate.
c) Eliminate protein interference → proteins are usually removed by precipitation or other sample prep methods, not by lanthanum.
31 / 60
Category:
ASCP Exam Questions
A decreased serum phosphate concentration may indicate:
A decreased serum phosphate concentration is known as hypophosphatemia . Vitamin D plays a crucial role in the intestinal absorption of both calcium and phosphate. Therefore, a deficiency in vitamin D leads to reduced absorption of phosphate from the diet, resulting in low serum phosphate levels.
Why the other options are incorrect:
b) Renal failure: This typically causes hyperphosphatemia (high phosphate) because the kidneys are unable to excrete phosphate effectively.
c) Acidosis: While acidosis can cause a shift of phosphate out of cells, it is not a primary cause of hypophosphatemia. The relationship is complex, but acidosis itself does not consistently indicate low phosphate.
d) Hypoparathyroidism: This condition is characterized by hyperphosphatemia (high phosphate) due to low levels of parathyroid hormone (PTH), which normally promotes phosphate excretion by the kidneys.
32 / 60
Category:
ASCP Exam Questions
The principal extracellular anion is:
Chloride (Cl⁻) is the most abundant anion in the extracellular fluid. It plays a crucial role in:
Maintaining electroneutrality alongside sodium.
Regulating osmotic pressure .
Facilitating acid-base balance through the chloride shift in red blood cells.
Why the other options are incorrect:
a) Bicarbonate (HCO₃⁻): This is the second most abundant extracellular anion and is vital for acid-base balance, but its concentration is lower than chloride.
c) Sulfate (SO₄²⁻) & d) Phosphate (HPO₄²⁻): These are primarily intracellular anions and are present in much lower concentrations in the extracellular fluid.
33 / 60
Category:
ASCP Exam Questions
Vitamin D is essential for:
Other options:
a) Wound healing → mainly requires Vitamin C
c) Collagen formation → Vitamin C
d) Red blood cell production → mainly Vitamin B12 and folate
34 / 60
Category:
ASCP Exam Questions
A low concentration of serum phosphorus is commonly found in:
Other options:
a) Chronic renal disease → usually causes hyperphosphatemia because the kidneys cannot excrete phosphate efficiently.
b) Hypoparathyroidism → usually associated with hyperphosphatemia due to reduced PTH-mediated phosphate excretion.
d) Pituitary tumors → not typically associated with hypophosphatemia.
35 / 60
Category:
ASCP Exam Questions
Which of the following findings is characteristic of iron deficiency anemia?
Iron deficiency anemia (IDA) has a characteristic pattern on iron studies due to the body’s physiological response to a lack of iron:
Low Serum Iron: There is an insufficient amount of iron circulating in the blood bound to transferrin.
High Total Iron-Binding Capacity (TIBC): The body attempts to compensate for the iron deficiency by increasing the production of transferrin, the iron-transport protein. This increases the blood’s total capacity to bind iron, resulting in a high TIBC.
This combination effectively distinguishes IDA from other microcytic anemias, particularly Anemia of Chronic Disease , which shows a Low serum iron, Low TIBC pattern.
Why the other options are incorrect:
b) High serum iron, Low TIBC: This is the pattern seen in iron overload conditions like hemochromatosis .
c) Low serum iron, Low TIBC: This is the classic pattern for Anemia of Chronic Disease .
d) High serum iron, High TIBC: This is not a typical pattern for common iron disorders. It could theoretically be seen with recent iron ingestion or in some cases of hemolytic anemia, but it is not characteristic of IDA.
36 / 60
Category:
ASCP Exam Questions
A decreased serum calcium level is known as:
The term is derived from:
Therefore, hypocalcemia specifically means a low level of calcium in the blood .
Why the other options are incorrect:
a) Hypercalcemia: This means a high level of calcium in the blood.
c) Hypokalemia: This means a low level of potassium in the blood.
d) Hyponatremia: This means a low level of sodium in the blood.
37 / 60
Category:
ASCP Exam Questions
The term “hyponatremia” is derived from:
Therefore, hyponatremia specifically means a low concentration of sodium in the blood .
Why the other options are incorrect:
a) Low blood potassium: This is called hypokalemia .
c) Low blood chloride: This is called hypochloremia .
d) Low blood calcium: This is called hypocalcemia .
38 / 60
Category:
ASCP Exam Questions
Megaloblastic anemia is commonly caused by a deficiency of:
Megaloblastic anemia is characterized by the production of abnormally large, immature, and dysfunctional red blood cells called megaloblasts in the bone marrow. This occurs due to impaired DNA synthesis.
Both Folic acid (Vitamin B9) and Vitamin B12 (Cobalamin) are essential for the synthesis of thymidine, a key building block of DNA. A deficiency in either vitamin disrupts DNA production, causing the red blood cell precursors to grow large but fail to divide properly, leading to megaloblastic anemia.
Why the other options are incorrect:
b) Thiamine (B1): Deficiency causes beriberi, not megaloblastic anemia.
c) Riboflavin (B2): Deficiency causes ariboflavinosis, not megaloblastic anemia.
d) Vitamin K: Deficiency causes a bleeding disorder due to impaired synthesis of clotting factors, not megaloblastic anemia.
39 / 60
Category:
ASCP Exam Questions
Magnesium plays a key role in:
Magnesium is a crucial cofactor for hundreds of enzymes and is essential for:
Enzyme Activity: It is required for the activity of many enzymes involved in:
ATP metabolism: Mg²⁺ complexes with ATP, forming MgATP²⁻, which is the true substrate for kinases and other ATP-dependent enzymes.
Carbohydrate metabolism
Nucleic Acid Synthesis: It is essential for the function of DNA and RNA polymerases.
DNA/RNA Stability: Mg²⁺ helps stabilize the structure of DNA and RNA by neutralizing the negative charge of the phosphate backbone.
Membrane and Bone Structure: It is also important for bone formation and neuromuscular function.
Why the other options are incomplete or incorrect:
a) Protein synthesis only: This is too narrow. While Mg²⁺ is involved in protein synthesis (as a ribosome cofactor), its role is far broader.
c) Bone resorption: This process is primarily regulated by parathyroid hormone (PTH). Magnesium is a component of bone matrix, but its key role is not in resorption.
d) Bile acid metabolism: This is not a primary function of magnesium.
40 / 60
Category:
ASCP Exam Questions
A patient’s laboratory results show: low serum iron and low TIBC. This pattern is most consistent with:
Comparison with other options:
a) Iron Deficiency Anemia: Low serum iron with high TIBC (transferrin rises to transport more iron).
c) Hemochromatosis: High serum iron and high ferritin.
d) Thalassemia: Variable iron parameters; TIBC is usually normal.
41 / 60
Category:
ASCP Exam Questions
The major intracellular cation in the body is:
The distribution of major cations across cell membranes is highly regulated:
Potassium (K⁺) is the major intracellular cation . Its high concentration inside cells (about 140-150 mmol/L) is maintained by the Na⁺/K⁺ ATPase pump and is critical for maintaining the resting membrane potential, cell volume, and various cellular functions.
Sodium (Na⁺) is the major extracellular cation .
Why the other options are incorrect:
a) Sodium: This is the primary cation outside cells (in the extracellular fluid).
b) Calcium: While calcium is a vital intracellular signaling molecule, its concentration inside the cell is kept very low (~10,000 times lower than extracellular levels). It is not the major intracellular cation by concentration.
d) Chloride: This is an anion , not a cation, and is primarily extracellular.
42 / 60
Category:
ASCP Exam Questions
Which of the following vitamins is water-soluble?
Vitamins are classified into two main groups based on their solubility:
Water-Soluble Vitamins: These dissolve in water and are not stored in the body to a significant extent. They include the B-complex vitamins (such as thiamine, riboflavin, niacin, B6, B12, and folate) and Vitamin C .
Fat-Soluble Vitamins: These dissolve in fats and oils and are stored in the body’s liver and fatty tissues. They include Vitamins A, D, E, and K .
Why the other options are incorrect:
a) Vitamin A: This is a fat-soluble vitamin.
c) Vitamin D: This is a fat-soluble vitamin.
d) Vitamin K: This is a fat-soluble vitamin.
43 / 60
Category:
ASCP Exam Questions
Wilson’s disease, characterized by abnormal copper accumulation, is associated with:
Wilson’s disease is an autosomal recessive disorder caused by a mutation in the ATP7B gene, leading to impaired copper incorporation into ceruloplasmin and reduced biliary copper excretion.
Why the other options are incorrect:
a) Increased serum copper and decreased urine copper: This is the opposite of the Wilson’s disease profile.
c) Increased serum ceruloplasmin: Ceruloplasmin is decreased, not increased.
d) Decreased urine copper: Urine copper is characteristically increased, not decreased.
44 / 60
Category:
ASCP Exam Questions
The osmolal gap is defined as the difference between:
Calculated osmolality formula (approximate):
Calculated Osmolality (mOsm/kg)=2[Na⁺]+Glucose18+BUN2.8\text{Calculated Osmolality (mOsm/kg)} = 2[\text{Na⁺}] + \frac{\text{Glucose}}{18} + \frac{\text{BUN}}{2.8} Calculated Osmolality (mOsm/kg) = 2 [ Na⁺ ] + 18Glucose + 2.8BUN
Other options:
a) Ideal and real osmolality values → not standard terminology.
c) Plasma and water osmolality → irrelevant.
d) Urine and serum osmolality → used in renal concentrating ability, not osmolal gap.
45 / 60
Category:
ASCP Exam Questions
Beriberi is due to a deficiency of:
Beriberi is caused by a deficiency of thiamine (Vitamin B1) , which is a coenzyme in carbohydrate metabolism (e.g., pyruvate dehydrogenase, α-ketoglutarate dehydrogenase).
Clinical forms:
Dry beriberi → peripheral neuropathy
Wet beriberi → high-output cardiac failure, edema
Other options:
Vitamin C → scurvy
Vitamin B6 (Pyridoxine) → peripheral neuropathy, sideroblastic anemia
Vitamin B12 → pernicious anemia, neurological deficits
46 / 60
Category:
ASCP Exam Questions
Approximately 90% of the copper present in the blood is bound to:
Ceruloplasmin is a copper-carrying glycoprotein produced by the liver.
It binds ~90% of copper in plasma and transports it to tissues.
It also has oxidase activity , assisting in iron metabolism by oxidizing Fe²⁺ to Fe³⁺ for binding to transferrin.
Other options:
a) Transferrin → carries iron , not copper.
b) Albumin → binds a small fraction (~5–10%) of copper; not the major carrier.
d) Cryoglobulin → not related to copper transport.
47 / 60
Category:
ASCP Exam Questions
Vitamin K is required for the synthesis of:
Vitamin K is essential for the post-translational gamma-carboxylation of glutamic acid residues on certain proteins.
This modification is required for the biological activity of clotting factors :
Factor II (prothrombin)
Factor VII
Factor IX
Factor X
Without Vitamin K, these clotting factors are inactive , leading to bleeding tendencies .
Other options:
a) Hemoglobin → requires iron, Vitamin B12, and folate
c) Albumin → synthesized in the liver; not Vitamin K dependent
d) Collagen → requires Vitamin C
48 / 60
Category:
ASCP Exam Questions
The regulation of calcium and phosphorus metabolism is primarily accomplished by which gland?
The parathyroid glands (usually four small glands located behind the thyroid) regulate calcium and phosphate metabolism primarily through parathyroid hormone (PTH) .
Why the other options are incorrect:
a) Thyroid: The thyroid gland produces calcitonin, which can lower blood calcium levels, but its role in adult humans is minor compared to PTH. The thyroid’s primary function is to regulate metabolism via T3 and T4.
c) Adrenal Glands: These glands regulate electrolytes like sodium and potassium (via aldosterone) and the stress response (via cortisol), not calcium and phosphorus.
d) Pituitary: This is the “master gland” that regulates other endocrine glands (like the thyroid, adrenals, and gonads), but it does not directly control calcium and phosphorus metabolism.
49 / 60
Category:
ASCP Exam Questions
The main buffer system that maintains blood pH is the:
The bicarbonate (HCO₃⁻) – carbonic acid (H₂CO₃) system is the primary extracellular buffer that maintains blood pH around 7.35–7.45 . It works in coordination with lungs (CO₂ removal) and kidneys (HCO₃⁻ regulation) .
Why the other options are incorrect:
a) Phosphate buffer (HPO₄²⁻/H₂PO₄⁻): This is an important buffer system, but its main role is in the intracellular fluid and in the urine within the kidneys’ tubular fluid. Its concentration in the blood is too low to be the primary buffer.
b) Hemoglobin buffer: This is a crucial buffer inside red blood cells for handling CO₂ and the H⁺ ions generated from carbonic acid. However, it is not the main buffer for the blood plasma as a whole.
d) Protein buffer: Proteins (especially albumin) contribute to the buffering capacity of the blood, but they are secondary to the bicarbonate system.
50 / 60
Category:
ASCP Exam Questions
Which of the following is a water-soluble vitamin?
Vitamins are categorized based on their solubility:
Water-Soluble Vitamins: These dissolve in water and are not stored in the body to a significant extent. They include Vitamin C and all the B-complex vitamins (such as thiamine, riboflavin, niacin, B6, B12, and folate).
Fat-Soluble Vitamins: These dissolve in fats and oils and are stored in the body’s liver and fatty tissues. They include Vitamins A, D, E, and K .
Why the other options are incorrect:
a) Vitamin D: This is a fat-soluble vitamin.
b) Vitamin K: This is a fat-soluble vitamin.
c) Vitamin E: This is a fat-soluble vitamin.
51 / 60
Category:
ASCP Exam Questions
A deficiency of which vitamin leads to night blindness?
Vitamin A (retinol) is essential for the synthesis of rhodopsin , the photopigment found in the rods of the retina. Rods are responsible for vision in low-light conditions. A deficiency of vitamin A impairs the regeneration of rhodopsin after exposure to light, leading to nyctalopia (night blindness) , which is one of the earliest and most characteristic symptoms of vitamin A deficiency.
Why the other options are incorrect:
b) Vitamin C: A deficiency causes scurvy , characterized by bleeding gums, poor wound healing, and weakened connective tissues.
c) Niacin (Vitamin B3): A deficiency causes pellagra , characterized by the “4 Ds”: dermatitis, diarrhea, dementia, and death.
d) Thiamine (Vitamin B1): A deficiency causes beriberi (affecting the cardiovascular and nervous systems) and Wernicke-Korsakoff syndrome .
52 / 60
Category:
ASCP Exam Questions
Pernicious anemia is associated with a deficiency of:
Pernicious anemia is a specific form of megaloblastic anemia caused by the malabsorption of vitamin B12 (cobalamin) due to a lack of intrinsic factor .
Intrinsic factor is a glycoprotein produced by the parietal cells of the stomach.
In pernicious anemia, the body’s immune system attacks and destroys these parietal cells, leading to a deficiency of intrinsic factor.
Without intrinsic factor, dietary vitamin B12 cannot be absorbed in the ileum, leading to a deficiency.
Vitamin B12 is essential for DNA synthesis and red blood cell formation. Its deficiency causes the production of large, immature, and dysfunctional red blood cells (megaloblasts).
Why the other options are incorrect:
a) Vitamin A: Deficiency causes night blindness and xerophthalmia, not pernicious anemia.
c) Niacin (Vitamin B3): Deficiency causes pellagra.
d) Thiamine (Vitamin B1): Deficiency causes beriberi and Wernicke-Korsakoff syndrome.
53 / 60
Category:
ASCP Exam Questions
The most accurate method for measuring ionized calcium involves the use of a(n):
Ionized calcium (Ca²⁺) is the physiologically active fraction of serum calcium.
The most accurate measurement is performed using an ion-selective electrode (ISE) that specifically measures free Ca²⁺ ions in plasma or serum.
This is important because total calcium may be misleading in cases of hypoalbuminemia or acid-base disturbances .
Other methods:
a) Colorimetric assay with o-cresolphthalein complexone → measures total calcium , not ionized calcium.
c) Atomic absorption spectrophotometer → measures total elemental calcium; not routinely used for ionized calcium.
d) Fluorometric assay → less commonly used; less precise for direct ionized calcium measurement.
54 / 60
Category:
ASCP Exam Questions
Absorption of dietary vitamin B12 requires the presence of:
Vitamin B12 (cobalamin) is absorbed in the terminal ileum .
Intrinsic Factor (IF) is a glycoprotein produced by gastric parietal cells that binds B12 and protects it from digestion, allowing absorption.
Other options:
a) Gastrin → stimulates acid secretion; not directly required for B12 absorption.
c) Secretin → stimulates pancreatic bicarbonate; not required for B12 absorption.
d) Folic Acid → another vitamin; not involved in B12 absorption.
55 / 60
Category:
ASCP Exam Questions
The principal extracellular anion is:
Chloride (Cl⁻) is the most abundant anion in the extracellular fluid. It plays a crucial role in:
Maintaining electroneutrality alongside sodium.
Regulating osmotic pressure .
Facilitating acid-base balance through the chloride shift in red blood cells.
Why the other options are incorrect:
a) Bicarbonate (HCO₃⁻): This is the second most abundant extracellular anion and is vital for acid-base balance, but its concentration is lower than chloride.
c) Sulfate (SO₄²⁻) & d) Phosphate (HPO₄²⁻): These are primarily intracellular anions and are present in much lower concentrations in the extracellular fluid.
56 / 60
Category:
ASCP Exam Questions
The major extracellular cation is:
Potassium is the major intracellular cation , essential for maintaining cell membrane potential, nerve impulse transmission, and muscle contraction.
Sodium is primarily an extracellular cation .
Calcium is mostly stored in bones and has signaling roles.
Chloride is mainly an extracellular anion .
57 / 60
Category:
ASCP Exam Questions
The normal reference range for serum potassium is approximately:
The normal reference range for serum potassium in adults is typically 3.5 to 5.0 millimoles per liter (mmol/L) . This narrow range is critically important for maintaining normal neuromuscular function and cardiac rhythm.
Why the other options are incorrect:
a) 2.0–3.0 mmol/L: This represents severe, life-threatening hypokalemia .
c) 6.0–8.0 mmol/L: This represents moderate to severe hyperkalemia , which can cause fatal cardiac arrhythmias.
d) 8.0–10.0 mmol/L: This range is incompatible with life due to cardiac arrest.
58 / 60
Category:
ASCP Exam Questions
Which electrolyte imbalance is most likely associated with cardiac arrhythmia?
Hypokalemia (low potassium) can disrupt the electrical activity of the heart , leading to arrhythmias such as premature beats, tachycardia, or even ventricular fibrillation .
Hypernatremia primarily affects neurological function .
Hypochloremia affects acid-base balance but rarely directly causes arrhythmias.
Hypercalcemia can cause cardiac effects but is less commonly associated with arrhythmias than potassium disturbances.
59 / 60
Category:
ASCP Exam Questions
Which vitamin deficiency causes scurvy?
Vitamin C (ascorbic acid) is essential for hydroxylation of proline and lysine during collagen synthesis .
Deficiency leads to scurvy , characterized by:
Bleeding gums
Easy bruising
Poor wound healing
Petechiae
Other options:
Vitamin D → rickets/osteomalacia
Vitamin A → night blindness
Vitamin K → bleeding due to defective clotting factors
60 / 60
Category:
ASCP Exam Questions
Pellagra, characterized by dermatitis, diarrhea, and dementia, is caused by a deficiency of:
Pellagra is a disease caused by a deficiency of niacin (vitamin B3) or its precursor, the amino acid tryptophan. Its classic presentation is known as the “3 Ds” :
Dermatitis: A characteristic symmetrical, pigmented rash on skin exposed to sunlight.
Diarrhea: Inflammation of the mucous membranes of the digestive tract.
Dementia: Neurological symptoms including confusion, disorientation, and memory loss.
If left untreated, it can lead to a fourth “D”: Death .
Why the other options are incorrect:
a) Vitamin A: Deficiency causes night blindness and xerophthalmia.
b) Vitamin B1 (Thiamine): Deficiency causes beriberi and Wernicke-Korsakoff syndrome.
d) Vitamin C: Deficiency causes scurvy.
Your score is
The average score is 67%
Follow us on Sicial Media:
Restart quiz
Top 8 Medical Laboratory Scientist (MLS) Exams: Top 8 Medical Laboratory Scientist (MLS) Exams that are recognized globally and can help professionals validate their credentials and enhance their career opportunities:
1. ASCP – American Society for Clinical Pathology (USA) Exam Name: MLS(ASCP)Eligibility: Bachelor’s degree with clinical laboratory experience.Global Recognition: HighPurpose: Certifies Medical Laboratory Scientists in the United States and internationally.2. AMT – American Medical Technologists (USA) Exam Name: MLT(AMT) or MT(AMT)Eligibility: Academic and/or work experience in medical laboratory technology.Global Recognition: ModeratePurpose: Credentialing for medical technologists and technicians.3. AIMS – Australian Institute of Medical and Clinical Scientists Exam Name: AIMS Certification ExamEligibility: Assessment of qualifications and work experience.Recognition: Required for practice in Australia.Purpose: Certification and registration in Australia.4. CSMLS – Canadian Society for Medical Laboratory Science Exam Name: CSMLS General or Subject-specific ExamsEligibility: Graduation from a CSMLS-accredited program or equivalent.Recognition: CanadaPurpose: Entry-to-practice certification in Canada.5. IBMS – Institute of Biomedical Science (UK) Exam Name: Registration and Specialist Portfolio AssessmentEligibility: Accredited degree and lab experience.Recognition: UK and some Commonwealth countries.Purpose: Biomedical Scientist registration with the HCPC (UK).6. HAAD / DOH – Department of Health, Abu Dhabi (UAE) Exam Name: DOH/HAAD License ExamEligibility: Degree in medical laboratory science and experience.Recognition: UAE (Abu Dhabi)Purpose: Licensure for medical laboratory practice in Abu Dhabi.7. DHA – Dubai Health Authority (UAE) Exam Name: DHA License Exam for Medical Laboratory TechnologistsEligibility: Relevant degree and experience.Recognition: Dubai, UAEPurpose: Professional license for clinical laboratory practice in Dubai.8. MOH – Ministry of Health (Gulf Countries like UAE, Saudi Arabia, Kuwait) Exam Name: MOH License ExamEligibility: BSc/Diploma in Medical Laboratory + experience.Recognition: Varies by country.Purpose: Required for practicing in public and private sector labs.Tags:
Possible References Used